Int J Stem Cells.
2023 Feb;16(1):27-35. 10.15283/ijsc22110.
Inhibition of Class I Histone Deacetylase Enhances Self-Reprogramming of Spermatogonial Stem Cells into Pluripotent Stem Cells
- Affiliations
-
- 1Department of Stem Cell Biology, Konkuk University School of Medicine, Seoul, Korea
- 2Center for Stem Cell Research, Institute of Advanced Biomedical Science, Konkuk University, Seoul, Korea
- 3Research Institute of Medical Science, Konkuk University, Seoul, Korea
- KMID: 2539618
- DOI: http://doi.org/10.15283/ijsc22110
Abstract
- Background and Objectives
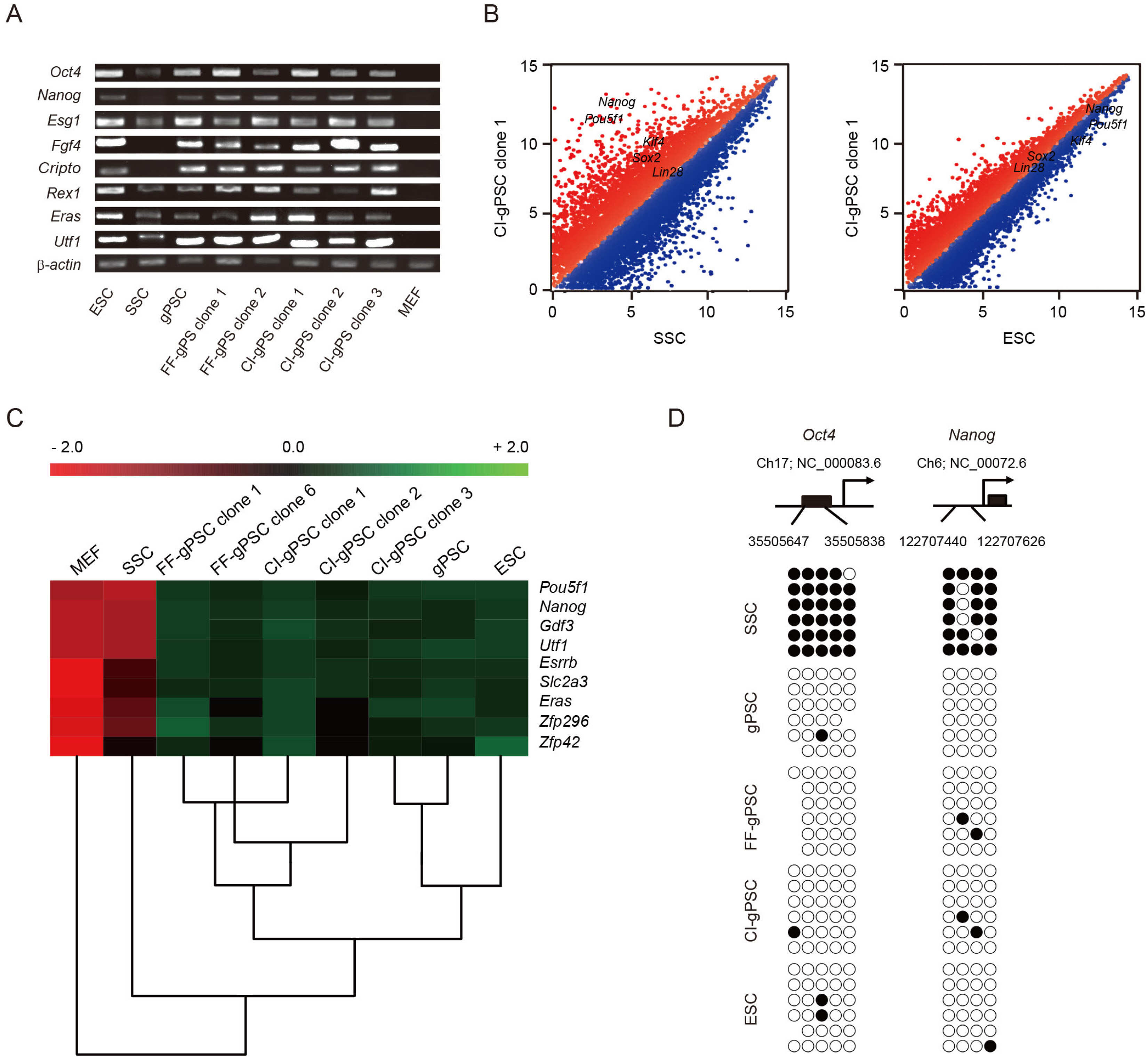
Spermatogonial stem cells (SSCs) are the most primitive cells in spermatogenesis and are the only adult stem cells capable of passing on the genome of a given species to the next generation. SSCs are the only adult stem cells known to exhibit high Oct4 expression and can be induced to self-reprogram into pluripotent cells depending on culture conditions. Epigenetic modulation is well known to be involved in the induction of pluripotency of somatic cells. However, epigenetic modulation in self-reprogramming of SSCs into pluripotent cells has not been studied.
Methods and Results
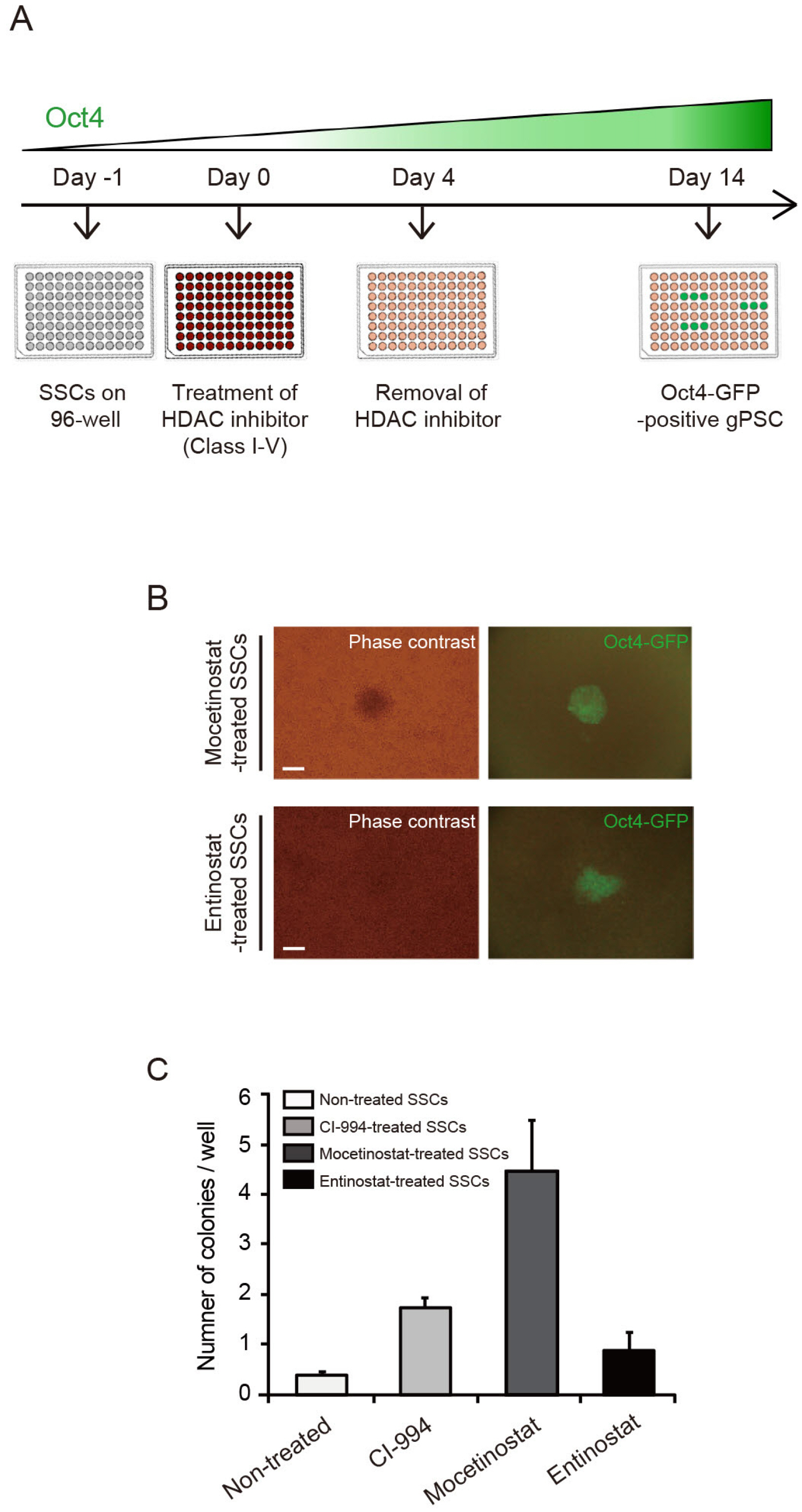
In this study, we examined the involvement of epigenetic modulation by assessing whether selfreprogramming of SSCs is enhanced by treatment with epigenetic modulators. We found that second-generation selective class I HDAC inhibitors increased SSC reprogramming efficiency, whereas non-selective HDAC inhibitors had no effect.
Conclusions
We showed that pluripotent stem cells derived from adult SSCs by treatment with small molecules with epigenetic modulator functions exhibit pluripotency in vitro and in vivo. Our results suggest that the mechanism of SSC reprogramming by epigenetic modulator can be used for important applications in epigenetic reprogramming research.
Keyword
Figure
Reference
-
References
1. Takahashi K, Yamanaka S. 2006; Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676. DOI: 10.1016/j.cell.2006.07.024. PMID: 16904174.2. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.3. Bernstein BE, Meissner A, Lander ES. 2007; The mammalian epigenome. Cell. 128:669–681. DOI: 10.1016/j.cell.2007.01.033. PMID: 17320505.4. Cedar H, Bergman Y. 2009; Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 10:295–304. DOI: 10.1038/nrg2540. PMID: 19308066.5. Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A. 2008; Dissecting direct reprogramming through integrative genomic analysis. Nature. 454:49–55. Erratum in: Nature 2008;454:794. DOI: 10.1038/nature07056. PMID: 18509334. PMCID: PMC2754827.6. Tegelenbosch RA, de Rooij DG. 1993; A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 290:193–200. DOI: 10.1016/0027-5107(93)90159-D. PMID: 7694110.7. Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. 2008; Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 26:2928–2937. DOI: 10.1634/stemcells.2008-0134. PMID: 18719224.8. Kanatsu-Shinohara M, Morimoto T, Toyokuni S, Shinohara T. 2004; Regulation of mouse spermatogonial stem cell self-rene-wing division by the pituitary gland. Biol Reprod. 70:1731–1737. DOI: 10.1095/biolreprod.103.025668. PMID: 14766726.9. Ko K, Tapia N, Wu G, Kim JB, Bravo MJ, Sasse P, Glaser T, Ruau D, Han DW, Greber B, Hausdörfer K, Sebastiano V, Stehling M, Fleischmann BK, Brüstle O, Zenke M, Schöler HR. 2009; Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 5:87–96. DOI: 10.1016/j.stem.2009.05.025. PMID: 19570517.10. Pesce M, Wang X, Wolgemuth DJ, Schöler H. 1998; Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 71:89–98. DOI: 10.1016/S0925-4773(98)00002-1. PMID: 9507072.11. Ko K, Araúzo-Bravo MJ, Kim J, Stehling M, Schöler HR. 2010; Conversion of adult mouse unipotent germline stem cells into pluripotent stem cells. Nat Protoc. 5:921–928. DOI: 10.1038/nprot.2010.44. PMID: 20431537.12. Lee SW, Wu G, Choi NY, Lee HJ, Bang JS, Lee Y, Lee M, Ko K, Schöler HR, Ko K. 2018; Self-reprogramming of spermatogonial stem cells into pluripotent stem cells without microenvironment of feeder cells. Mol Cells. 41:631–638. DOI: 10.14348/molcells.2018.2294. PMID: 29991673. PMCID: PMC6078851.13. Bang JS, Choi NY, Lee M, Ko K, Park YS, Ko K. 2019; Reprogramming of cancer cells into induced pluripotent stem cells questioned. Int J Stem Cells. 12:430–439. DOI: 10.15283/ijsc19067. PMID: 31474029. PMCID: PMC6881048.14. Ko K, Wu G, Araúzo-Bravo MJ, Kim J, Francine J, Greber B, Mühlisch J, Joo JY, Sabour D, Frühwald MC, Tapia N, Schöler HR. 2012; Autologous pluripotent stem cells generated from adult mouse testicular biopsy. Stem Cell Rev Rep. 8:435–444. DOI: 10.1007/s12015-011-9307-x. PMID: 21858421.15. Choi NY, Park YS, Ryu JS, Lee HJ, Araúzo-Bravo MJ, Ko K, Han DW, Schöler HR, Ko K. 2014; A novel feeder-free culture system for expansion of mouse spermatogonial stem cells. Mol Cells. 37:473–479. DOI: 10.14348/molcells.2014.0080. PMID: 24854861. PMCID: PMC4086341.16. Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. 2013; Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 341:651–654. DOI: 10.1126/science.1239278. PMID: 23868920.17. Brüstle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. 1999; Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 285:754–756. DOI: 10.1126/science.285.5428.754. PMID: 10427001.18. Igelmund P, Fleischmann BK, Fischer IR, Soest J, Gryshchenko O, Böhm-Pinger MM, Sauer H, Liu Q, Hescheler J. 1999; Action potential propagation failures in long-term recor-dings from embryonic stem cell-derived cardiomyocytes in tissue culture. Pflugers Arch. 437:669–679. DOI: 10.1007/s004240050831. PMID: 10087143.19. Lee HJ, Choi NY, Lee SW, Ko K, Hwang TS, Han DW, Lim J, Schöler HR, Ko K. 2016; Epigenetic alteration of imprinted genes during neural differentiation of germline-derived pluripotent stem cells. Epigenetics. 11:177–183. DOI: 10.1080/15592294.2016.1146852. PMID: 26962997. PMCID: PMC4854545.20. Kim KP, Han DW, Kim J, Schöler HR. 2021; Biological importance of OCT transcription factors in reprogramming and development. Exp Mol Med. 53:1018–1028. DOI: 10.1038/s12276-021-00637-4. PMID: 34117345. PMCID: PMC8257633.21. Federation AJ, Bradner JE, Meissner A. 2014; The use of small molecules in somatic-cell reprogramming. Trends Cell Biol. 24:179–187. DOI: 10.1016/j.tcb.2013.09.011. PMID: 24183602. PMCID: PMC3943685.22. Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. 2008; Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 26:795–797. DOI: 10.1038/nbt1418. PMID: 18568017. PMCID: PMC6334647.23. Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. 2008; Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 26:1269–1275. DOI: 10.1038/nbt.1502. PMID: 18849973.24. Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. 2008; Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 3:568–574. DOI: 10.1016/j.stem.2008.10.004. PMID: 18983970.25. Zhao Y, Zhao T, Guan J, Zhang X, Fu Y, Ye J, Zhu J, Meng G, Ge J, Yang S, Cheng L, Du Y, Zhao C, Wang T, Su L, Yang W, Deng H. 2015; A XEN-like state bridges somatic cells to pluripotency during chemical reprogramming. Cell. 163:1678–1691. DOI: 10.1016/j.cell.2015.11.017. PMID: 26686652.26. Staszkiewicz J, Power RA, Harkins LL, Barnes CW, Strickler KL, Rim JS, Bondioli KR, Eilersten KJ. 2013; Silencing histone deacetylase-specific isoforms enhances expression of pluripotency genes in bovine fibroblasts. Cell Reprogram. 15:397–404. DOI: 10.1089/cell.2013.0026. PMID: 24020699. PMCID: PMC3787336.27. Saha A, Pandian GN, Sato S, Taniguchi J, Hashiya K, Bando T, Sugiyama H. 2013; Synthesis and biological evaluation of a targeted DNA-binding transcriptional activator with HDAC8 inhibitory activity. Bioorg Med Chem. 21:4201–4209. DOI: 10.1016/j.bmc.2013.05.002. PMID: 23719282.28. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. 2003; Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 370(Pt 3):737–749. DOI: 10.1042/bj20021321. PMID: 12429021. PMCID: PMC1223209.29. Gregoretti IV, Lee YM, Goodson HV. 2004; Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 338:17–31. DOI: 10.1016/j.jmb.2004.02.006. PMID: 15050820.30. Haberland M, Montgomery RL, Olson EN. 2009; The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 10:32–42. DOI: 10.1038/nrg2485. PMID: 19065135. PMCID: PMC3215088.31. Kang J, Shakya A, Tantin D. 2009; Stem cells, stress, metabolism and cancer: a drama in two Octs. Trends Biochem Sci. 34:491–499. DOI: 10.1016/j.tibs.2009.06.003. PMID: 19733480.32. Pan GJ, Chang ZY, Schöler HR, Pei D. 2002; Stem cell pluripotency and transcription factor Oct4. Cell Res. 12:321–329. DOI: 10.1038/sj.cr.7290134. PMID: 12528890.33. Shi G, Jin Y. 2010; Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res Ther. 1:39. DOI: 10.1186/scrt39. PMID: 21156086. PMCID: PMC3025441.34. Tantin D. 2013; Oct transcription factors in development and stem cells: insights and mechanisms. Development. 140:2857–2866. DOI: 10.1242/dev.095927. PMID: 23821033. PMCID: PMC3699277.35. Wu G, Schöler HR. 2014; Role of Oct4 in the early embryo development. Cell Regen. 3:7. DOI: 10.1186/2045-9769-3-7. PMID: 25408886. PMCID: PMC4230828.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Valproic acid, a Histone Deacetylase Inhibitor, on the Expression of Pluripotency and Neural Crest Specific Marker Genes in Murine Multipotent Skin Precursor Cells
- The Role of Histone Acetylation in Mesenchymal Stem Cell Differentiation
- Disease-specific pluripotent stem cells
- Modeling of Human Genetic Diseases Via Cellular Reprogramming
- Stem cells: general information and perspectives