Korean J Health Promot.
2022 Dec;22(4):183-193. 10.15384/kjhp.2022.22.4.183.
Prescription of Gastric Acid Secretion Inhibitors before and after the Withdrawal of Ranitidine
- Affiliations
-
- 1Department of Pharmaceutical Engineering, Cheongju University, Cheongju, Korea
- KMID: 2537438
- DOI: http://doi.org/10.15384/kjhp.2022.22.4.183
Abstract
- Background
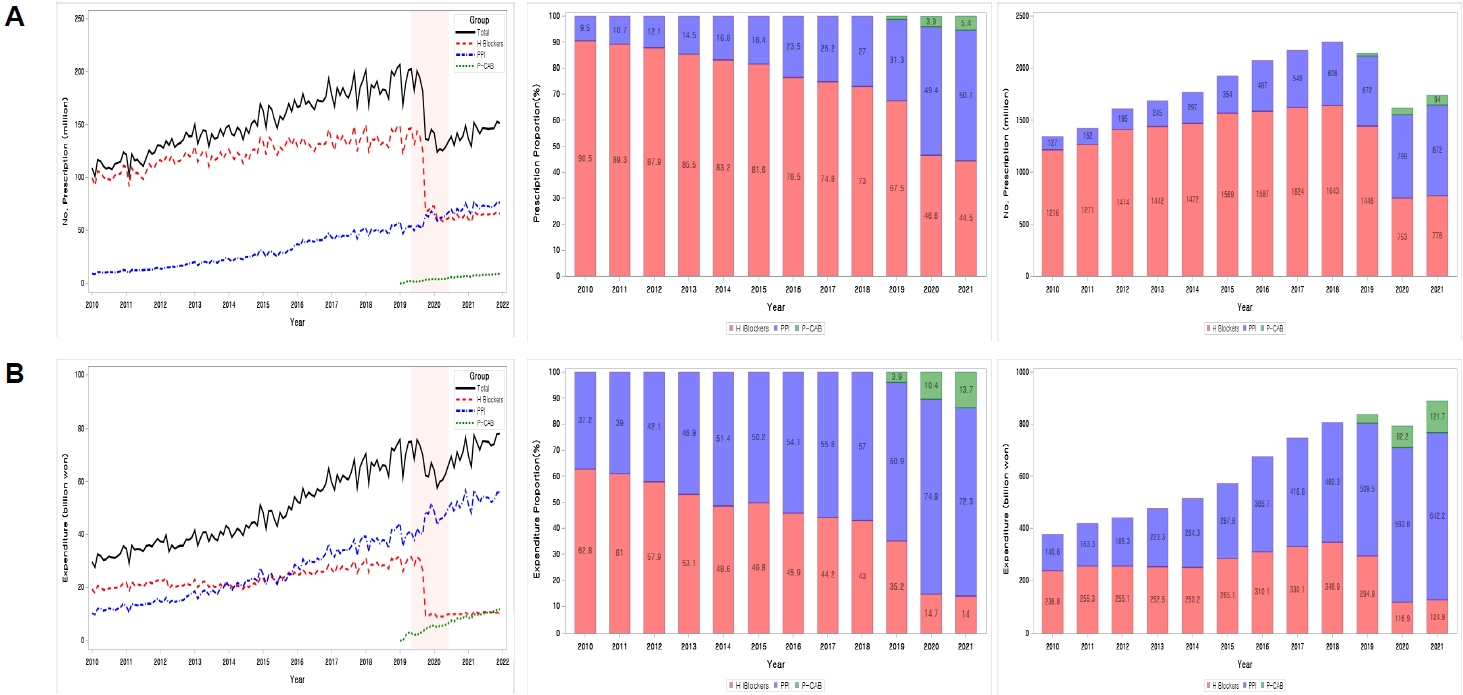
In September 2019, ranitidine, the largest share in the gastric acid secretion inhibitor market, was identified as a carcinogen, and sales were banned. The purpose of this study was to investigate how the gastric acid secretion inhibitor market changed after ranitidine withdrawal.
Methods
From January 2010 to December 2021, the prescription dose and cost of gastric acid secretion inhibitors were calculated monthly. To investigate the effect of ranitidine withdrawal on the gastric acid secretion inhibitor market, we developed a time-series autoregressive model using data from January 2010 to October 2019. In addition, the P-value was calculated by interrupted time series analysis using the data dating between 2010 and 2021 (interrupted time: October 2019).
Results
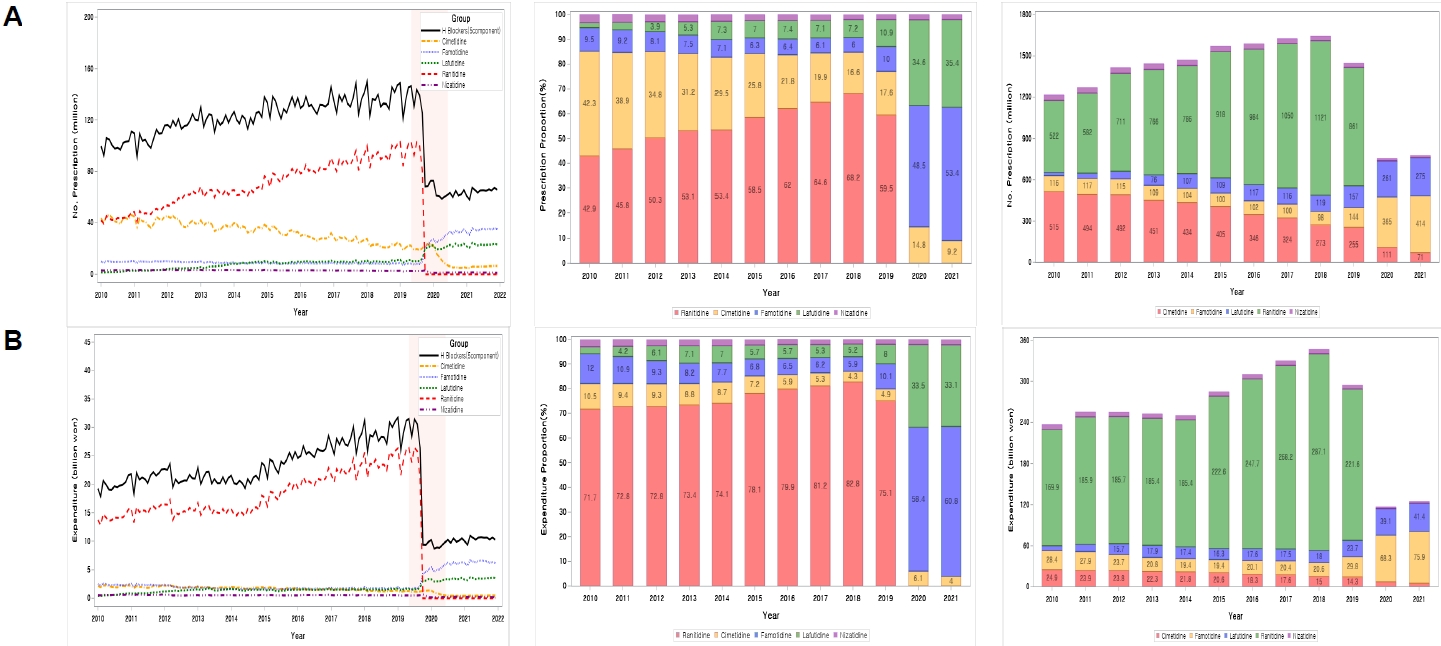
Since 2010, proton pump inhibitors have increased their market share in terms of prescription volume and drug costs. This trend accelerated since ranitidine was withdrawn from the market in September 2019. In 2021, it was estimated that ranitidine prescriptions would be transferred as follows: famotidine's increased prescription volume was estimated at 323 million (pharmaceutical cost, 53.2 billion won), proton pump inhibitors (PPIs) at 223 million (89.9 billion won), and lafutidine at 137 million (20.5 billion won).
Conclusions
The market share expansion of PPIs accelerated due to the withdrawal of ranitidine. The ranitidine prescription was partially transferred to the same H 2 blockers, such as famotidine and lafutidine, and there was also a significant transfer to PPIs.
Keyword
Figure
Reference
-
1. Yun J. Necessity of providing more detailed and specific information on drugs prohibited by the Ministry of Food and Drug Safety. Korean J Health Promot. 2020; 20(4):211–3.
Article2. Ministry of Food and Drug Safety. Temporary ban imposed on manufacture, import, and sale of ranitidine products [Internet]. Cheongju: Ministry of Food and Drug Safety;2019; [cited Jun 19, 2022]. Available from: https://www.mfds.go.kr/brd/m_99/view.do?seq=43717&srchFr=&srchTo=&srchWord=%EB%9D%BC%EB%8B%88%ED%8B%B0%EB%94%98&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=1.3. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl. 1987; 7:1–440.4. Sedlo I, Kolonić T, Tomić S. Presence of nitrosamine impurities in medicinal products. Arh Hig Rada Toksikol. 2021; 72(1):1–5.
Article5. Lim HH, Oh YS, Shin HS. Determination of N-nitrosodimethylamine and N-nitrosomethylethylamine in drug substances and products of sartans, metformin and ranitidine by precipitation and solid phase extraction and gas chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2020; 189:113460.
Article6. Shaik KM, Sarmah B, Wadekar GS, Kumar P. Regulatory updates and analytical methodologies for nitrosamine impurities detection in sartans, ranitidine, nizatidine, and metformin along with sample preparation techniques. Crit Rev Anal Chem. 2022; 52(1):53–71.
Article7. Aldawsari FS, Alshehry YM, Alghamdi TS. N-nitrosodimethylamine (NDMA) contamination of ranitidine products: a review of recent findings. J Food Drug Anal. 2021; 29(1):39–45.
Article8. Adamson RH, Chabner BA. The finding of N-nitrosodimethylamine in common medicines. Oncologist. 2020; 25(6):460–2.
Article9. U.S. Food and drug administration (FDA). FDA requests removal of all ranitidine products (zantac) from the market [Internet]. Silver Spring: FDA;2020. [cited Oct 12, 2022]. Available from: https://www.fda.gov/news-events/press-announcements/fdarequests-removal-all-ranitidine-products-zantacmarket.10. Ruepp R, Frötschl R, Bream R, Filancia M, Girard T, Spinei A, et al. The EU response to the presence of nitrosamine impurities in medicines. Front Med (Lausanne). 2021; 8:782536.
Article11. Health Sciences Authority (HAS). HSA stops supply of eight brands of ranitidine products in singapore [Internet]. Singapore: HAS;2019. [cited Oct 12, 2022]. Available from: https://www.hsa.gov.sg/announcements/news/hsa-stops-supply-of-eightbrands-of-ranitidine-products-in-singapore.12. Wagner JA, Dinh JC, Lightdale JR, Gold BD, Colombo JM. Is this the end for ranitidine? NDMA presence continues to confound. Clin Transl Sci. 2021; 14(4):1197–200.
Article13. Perisetti A, Goyal H, Tharian B. The 'burn' of ranitidine recall: current insights and mitigation strategies. Eur J Gastroenterol Hepatol. 2021; 33(1S Suppl 1):e1013–16.
Article14. Aschenbrenner DS. Ranitidine withdrawn from the market. Am J Nurs. 2020; 120(8):23.
Article15. White CM. Understanding and preventing (N-nitrosodimethylamine) NDMA contamination of medications. Ann Pharmacother. 2020; 54(6):611–4.
Article16. Wagner JA, Colombo JM. Medicine and media: the ranitidine debate. Clin Transl Sci. 2020; 13(4):649–51.
Article17. Yoon HJ, Kim JH, Seo GH, Park H. Risk of cancer following the use of N-nitrosodimethylamine (NDMA) contaminated ranitidine products: a nationwide cohort study in South Korea. J Clin Med. 2021; 10(1):153.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Different Route of Preanesthetic Ranitidine on Gastric Acidity in Patients Undergoing Elective Cesarean Section
- A Comparison of the Effects of Cimetidine, Ranitidine and Famotidine as Premedication on Gastric Volume and pH
- The Effect of H2-receptor in the Treatment of Peptic Ulcer
- Gastric pH Change according to the Administration Methods of Preanesthetic Ranitidine in Surgical Patient
- Effects of Ranitidine for Exercise Induced Gastric Mucosal Changes and Bleeding