Cardiovasc Prev Pharmacother.
2021 Jan;3(1):1-9. 10.36011/cpp.2021.3.e1.
Tafamidis for Cardiac Transthyretin Amyloidosis
- Affiliations
-
- 1Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2536946
- DOI: http://doi.org/10.36011/cpp.2021.3.e1
Abstract
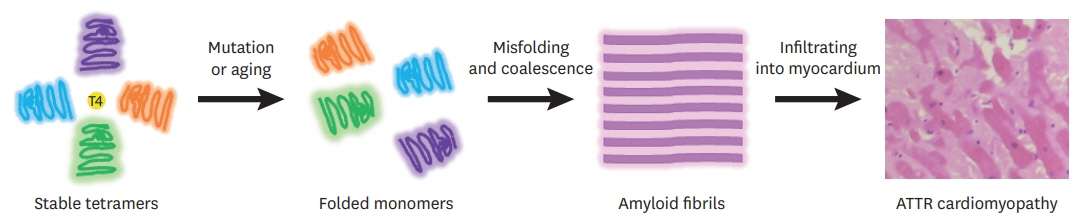
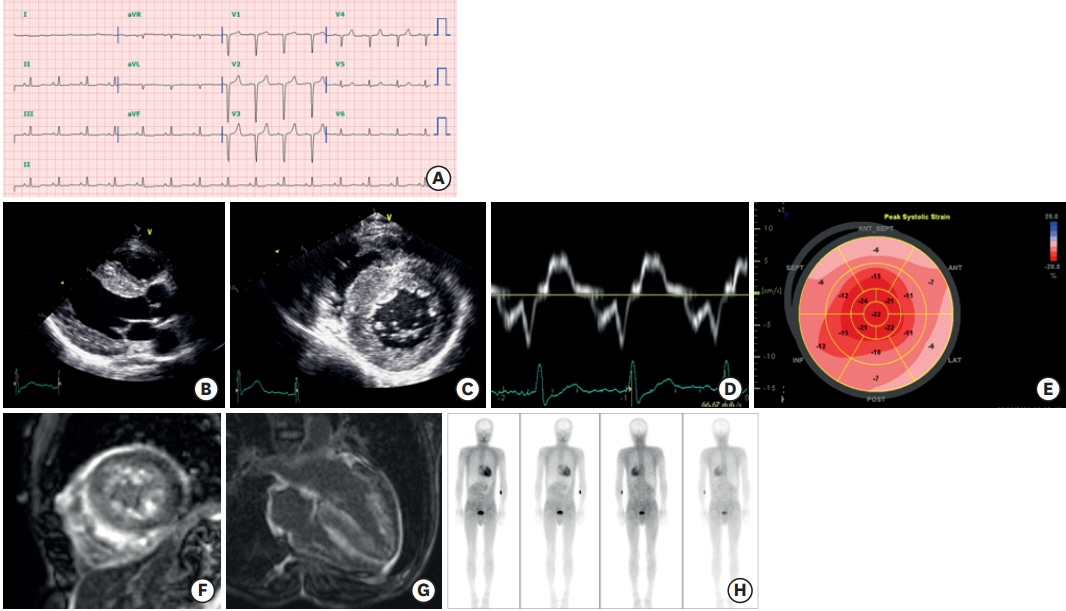
- Transthyretin amyloid (ATTR) cardiomyopathy is a progressive disease caused by the infiltration of ATTR fibrils in the myocardium. Although it is a rare disease, ATTR cardiomyopathy is an important cause of heart failure with preserved ejection fraction, and its incidence is increasing due to improved diagnostic imaging tools. There has been a breakthrough in the field of transthyretin amyloidosis, which opens a new therapeutic door for the patients. In this review, an overview of tafamidis therapy in ATTR cardiomyopathy with recent results from clinical trials will be discussed.
Keyword
Figure
Reference
-
1. Ando Y, Coelho T, Berk JL, Cruz MW, Ericzon BG, Ikeda S, Lewis WD, Obici L, Planté-Bordeneuve V, Rapezzi C, Said G, Salvi F. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013; 8:31.
Article2. Kato-Motozaki Y, Ono K, Shima K, Morinaga A, Machiya T, Nozaki I, Shibata-Hamaguchi A, Furukawa Y, Yanase D, Ishida C, Sakajiri K, Yamada M. Epidemiology of familial amyloid polyneuropathy in Japan: identification of a novel endemic focus. J Neurol Sci. 2008; 270:133–40.
Article3. Rapezzi C, Quarta CC, Obici L, Perfetto F, Longhi S, Salvi F, Biagini E, Lorenzini M, Grigioni F, Leone O, Cappelli F, Palladini G, Rimessi P, Ferlini A, Arpesella G, Pinna AD, Merlini G, Perlini S. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013; 34:520–8.
Article4. Hagen GA, Elliott WJ. Transport of thyroid hormones in serum and cerebrospinal fluid. J Clin Endocrinol Metab. 1973; 37:415–22.5. Rappley I, Monteiro C, Novais M, Baranczak A, Solis G, Wiseman RL, Helmke S, Maurer MS, Coelho T, Powers ET, Kelly JW. Quantification of transthyretin kinetic stability in human plasma using subunit exchange. Biochemistry. 2014; 53:1993–2006.
Article6. Liz MA, Mar FM, Franquinho F, Sousa MM. Aboard transthyretin: from transport to cleavage. IUBMB Life. 2010; 62:429–35.
Article7. Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019; 15:387–404.
Article8. Kelly JW. Amyloid fibril formation and protein misassembly: a structural quest for insights into amyloid and prion diseases. Structure. 1997; 5:595–600.
Article9. Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003; 349:583–96.
Article10. Rowczenio DM, Noor I, Gillmore JD, Lachmann HJ, Whelan C, Hawkins PN, Obici L, Westermark P, Grateau G, Wechalekar AD. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat. 2014; 35:E2403–12.
Article11. Purkey HE, Dorrell MI, Kelly JW. Evaluating the binding selectivity of transthyretin amyloid fibril inhibitors in blood plasma. Proc Natl Acad Sci U S A. 2001; 98:5566–71.
Article12. Zhao L, Buxbaum JN, Reixach N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry. 2013; 52:1913–26.
Article13. Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, Packman J, Powers ET, Wiseman RL, Foss TR, Wilson IA, Kelly JW; Labaudinière R. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012; 109:9629–34.
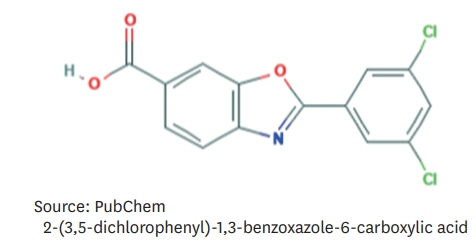
Article14. National Center for Biotechnology Information. PubChem compound summary for CID 11001318, tafamidis [Internet]. Bethesda, MD: National Center for Biotechnology Information;2020. [cited 2020 Dec 31]. Available from https://pubchem.ncbi.nlm.nih.gov/compound/Tafamidis.15. Coelho T, Merlini G, Bulawa CE, Fleming JA, Judge DP, Kelly JW, Maurer MS, Planté-Bordeneuve V, Labaudinière R, Mundayat R, Riley S, Lombardo I, Huertas P. Mechanism of action and clinical application of tafamidis in hereditary transthyretin amyloidosis. Neurol Ther. 2016; 5:1–25.
Article16. Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006; 13:236–49.
Article17. Schmidt HHJ. Tafamidis for the treatment of transthyretin-associated familial amyloid polyneuropathy. Expert Opin Orphan Drugs. 2013; 1:837–45.
Article18. Maurer MS, Sultan MB, Rapezzi C. Tafamidis for transthyretin amyloid cardiomyopathy. N Engl J Med. 2019; 380:196–7.
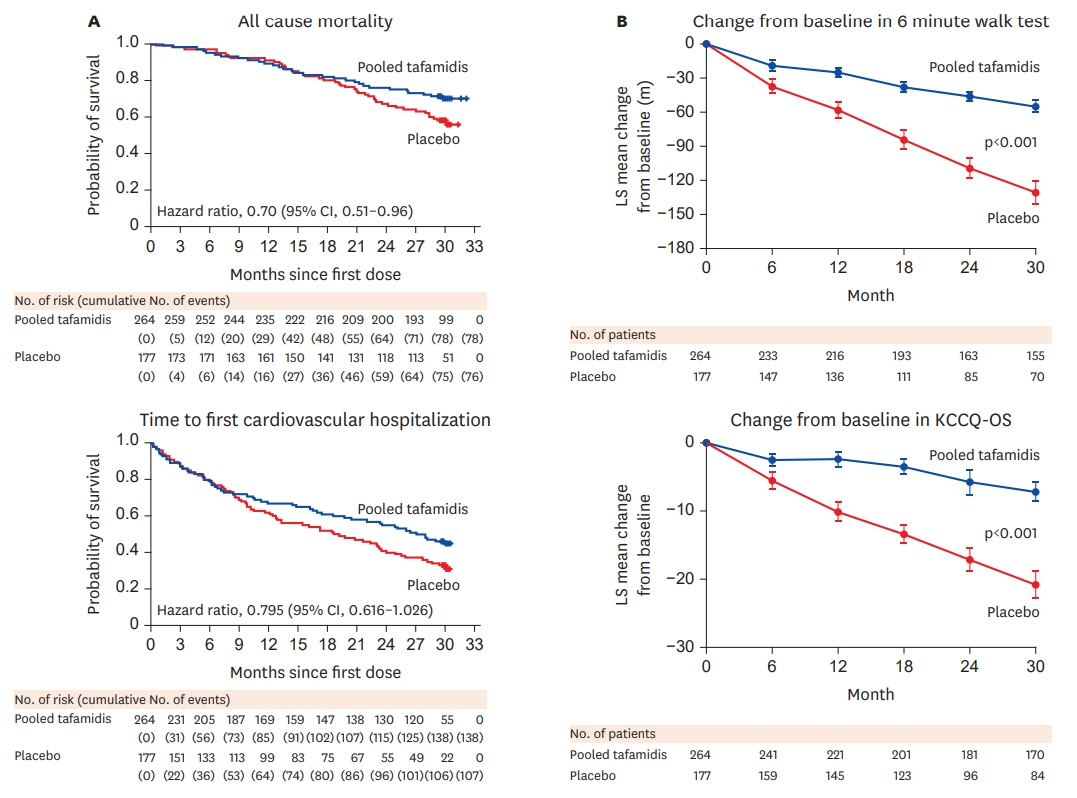
Article19. Maurer MS, Grogan DR, Judge DP, Mundayat R, Packman J, Lombardo I, Quyyumi AA, Aarts J, Falk RH. Tafamidis in transthyretin amyloid cardiomyopathy: effects on transthyretin stabilization and clinical outcomes. Circ Heart Fail. 2015; 8:519–26.20. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB; Rapezzi CATTR-ACT Study Investigators. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018; 379:1007–16.
Article21. Manion C, Sharma UC. Tafamidis for transthyretin amyloid cardiomyopathy. N Engl J Med. 2019; 380:196–7.
Article22. Damy T, Garcia-Pavia P, Hanna M, Judge DP, Merlini G, Gundapaneni B, Patterson TA, Riley S, Schwartz JH, Sultan MB, Witteles R. Efficacy and safety of tafamidis doses in the tafamidis in transthyretin cardiomyopathy clinical trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail. 2020; Oct. 18. [Epub ahead of print]. https://doi.org/10.1002/ejhf.2027.
Article23. Park J, Egolum U, Parker S, Andrews E, Ombengi D, Ling H. Tafamidis: a first-in-class transthyretin stabilizer for transthyretin amyloid cardiomyopathy. Ann Pharmacother. 2020; 54:470–7.
Article24. Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013; 369:819–29.
Article25. Benson MD, Ackermann EJ, Monia BP. Treatment of transthyretin cardiomyopathy with a TTR-specific antisense oligonucleotide (IONIS-TTRRx). Amyloid. 2017; 24:134–5.
Article26. Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA, Yeh RW, Arnold SV, Sperry BW, Maurer MS, Shah SJ. Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation. 2020; 141:1214–24.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transthyretin Cardiac Amyloidosis: A Case Report
- A rare pathogenic variant identified in a heart transplant recipient with hereditary transthyretin amyloidosis: a case report
- Hereditary Transthyretin Amyloidosis Misdiagnosed as Demyelinating Neuropathy: A Report of Three Cases
- Tafamidis, a Noninvasive Therapy for Delaying Transthyretin Familial Amyloid Polyneuropathy: Systematic Review and Meta-Analysis
- Familial Amyloidotic Polyneuropathy With Transthyretin Gene Mutation