Diabetes Metab J.
2022 Sep;46(5):713-721. 10.4093/dmj.2021.0164.
Glucose Profiles Assessed by Intermittently Scanned Continuous Glucose Monitoring System during the Perioperative Period of Metabolic Surgery
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2533640
- DOI: http://doi.org/10.4093/dmj.2021.0164
Abstract
- Background
Continuous glucose monitoring (CGM) has been widely used in the management of diabetes. However, the usefulness and detailed data during perioperative status were not well studied. In this study, we described the immediate changes of glucose profiles after metabolic surgery using intermittently scanned CGM (isCGM) in individuals with type 2 diabetes mellitus (T2DM).
Methods
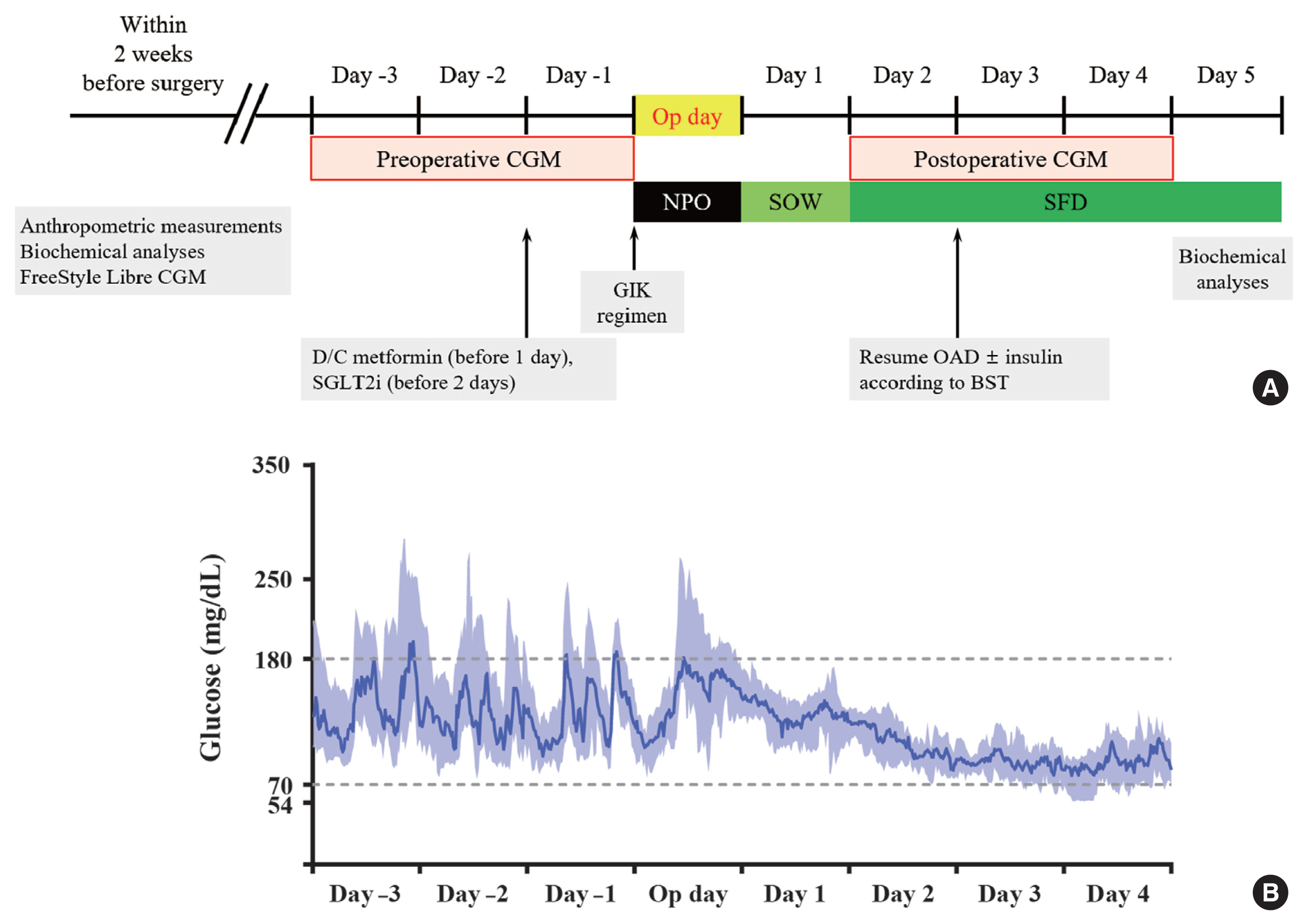
This was a prospective, single-center, single-arm study including 20 participants with T2DM. The isCGM (FreeStyle Libre CGM) implantation was performed within 2 weeks before surgery. We compared CGM metrics of 3 days before surgery and 3 days after surgery, and performed the correlation analyses with clinical variables.
Results
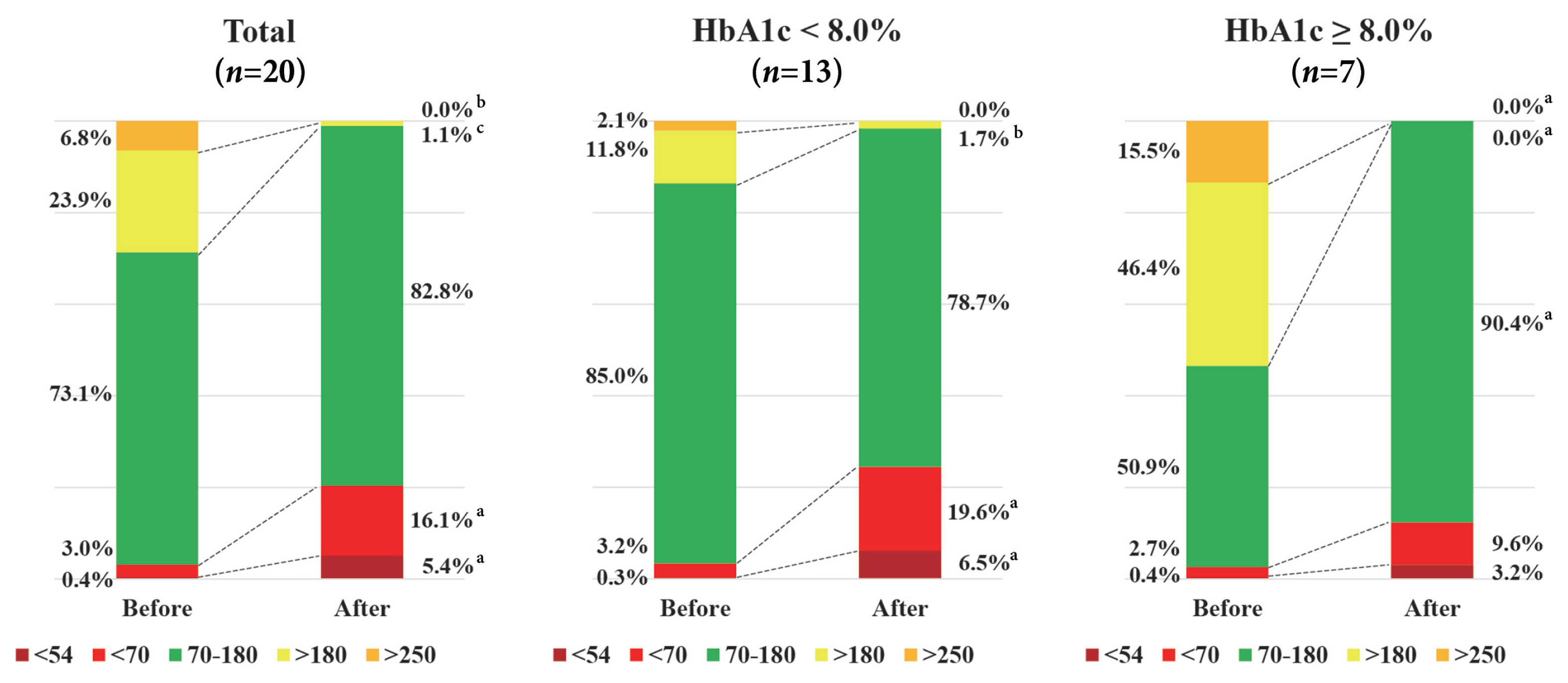
The mean glucose significantly decreased after surgery (147.0±40.4 to 95.5±17.1 mg/dL, P<0.001). Time in range (TIR; 70 to 180 mg/dL) did not significantly change after surgery in total. However, it was significantly increased in a subgroup of individuals with glycosylated hemoglobin (HbA1c) ≥8.0%. Time above range (>250 or 180 mg/dL) was significantly decreased in total. In contrast, time below range (<70 or 54 mg/dL) was significantly increased in total and especially in a subgroup of individuals with HbA1c <8.0% after surgery. The coefficient of variation significantly decreased after surgery. Higher baseline HbA1c was correlated with greater improvement in TIR (rho=0.607, P=0.005).
Conclusion
The isCGM identified improvement of mean glucose and glycemic variability, and increase of hypoglycemia after metabolic surgery, but TIR was not significantly changed after surgery. We detected an increase of TIR only in individuals with HbA1c ≥8.0%.
Figure
Cited by 2 articles
-
Asymptomatic Hypoglycemia after Metabolic Surgery: New Insights from Perioperative Continuous Glucose Monitoring
Sang-Man Jin
Diabetes Metab J. 2022;46(5):675-676. doi: 10.4093/dmj.2022.0307.Comparative Effect of Glucose-Lowering Drugs for Type 2 Diabetes Mellitus on Stroke Prevention: A Systematic Review and Network Meta-Analysis
Ji Soo Kim, Gyeongsil Lee, Kyung-Il Park, Seung-Won Oh
Diabetes Metab J. 2024;48(2):312-320. doi: 10.4093/dmj.2022.0421.
Reference
-
1. Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017; 14:160–9.
Article2. Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005; 15:474–81.
Article3. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it?: an operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995; 222:339–52.
Article4. Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, et al. Practical recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the post-bariatric surgery medical management. Obes Facts. 2017; 10:597–632.
Article5. Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications. 2017; 31:280–7.
Article6. Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J. 2019; 43:383–97.
Article7. Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018; 41:2265–74.
Article8. Yip S, Signal M, Smith G, Beban G, Booth M, Babor R, et al. Lower glycemic fluctuations early after bariatric surgery partially explained by caloric restriction. Obes Surg. 2014; 24:62–70.
Article9. Wysocki M, Szopa M, Stefura T, Dudek A, Torbicz G, Gajewska N, et al. Continuous glucose monitoring in bariatric patients undergoing laparoscopic sleeve gastrectomy and laparoscopic Roux-En-Y gastric bypass. Obes Surg. 2019; 29:1317–26.
Article10. Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the Guardian RT continuous glucose monitor in children with type 1 diabetes. Diabetes Technol Ther. 2008; 10:266–72.11. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019; 42:1593–603.12. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017; 40:1631–40.
Article13. Oh TJ, Kook JH, Jung SY, Kim DW, Choi SH, Kim HB, et al. A standardized glucose-insulin-potassium infusion protocol in surgical patients: use of real clinical data from a clinical data warehouse. Diabetes Res Clin Pract. 2021; 174:108756.
Article14. Sondergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2017; 102:1193–9.
Article15. Yoo JH, Kim JH. Time in range from continuous glucose monitoring: a novel metric for glycemic control. Diabetes Metab J. 2020; 44:828–39.
Article16. Schierenbeck F, Franco-Cereceda A, Liska J. Accuracy of 2 different continuous glucose monitoring systems in patients undergoing cardiac surgery. J Diabetes Sci Technol. 2017; 11:108–16.
Article17. Perez-Guzman MC, Duggan E, Gibanica S, Cardona S, Corujo-Rodriguez A, Faloye A, et al. Continuous glucose monitoring in the operating room and cardiac intensive care unit. Diabetes Care. 2021; 44:e50–2.
Article18. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015; 17:787–94.
Article19. Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmuhler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis. 2015; 11:564–9.
Article20. Hanaire H, Dubet A, Chauveau ME, Anduze Y, Fernandes M, Melki V, et al. Usefulness of continuous glucose monitoring for the diagnosis of hypoglycemia after a gastric bypass in a patient previously treated for type 2 diabetes. Obes Surg. 2010; 20:126–9.
Article21. Pleus S, Heinemann L, Freckmann G. Blood glucose monitoring data should be reported in detail when studies about efficacy of continuous glucose monitoring systems are published. J Diabetes Sci Technol. 2018; 12:1061–3.
Article22. Galindo RJ, Migdal AL, Davis GM, Urrutia MA, Albury B, Zambrano C, et al. Comparison of the FreeStyle Libre Pro Flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020; 43:2730–5.23. Hofso D, Fatima F, Borgeraas H, Birkeland KI, Gulseth HL, Hertel JK, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2019; 7:912–24.
Article24. Wang L, Shi C, Yan H, Xia M, Zhu X, Sun X, et al. Acute effects of sleeve gastrectomy on glucose variability, glucose metabolism, and ghrelin response. Obes Surg. 2021; 31:4005–14.
Article25. Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Chen SC, et al. Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis. 2013; 9:379–84.
Article26. Still CD, Wood GC, Benotti P, Petrick AT, Gabrielsen J, Strodel WE, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol. 2014; 2:38–45.
Article27. Bojsen-Moller KN, Dirksen C, Jorgensen NB, Jacobsen SH, Serup AK, Albers PH, et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014; 63:1725–37.
Article28. Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011; 54:2093–102.
Article29. Dagogo-Jack S, Alberti KG. Management of diabetes mellitus in surgical patients. Diabetes Spectr. 2002; 15:44–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Continuous Glucose Monitoring System (CGMS) and Patient Education
- Glucose Management Using Continuous Glucose Monitors
- Importance of continuous glucose monitoring in the treatment of diabetes mellitus
- Asymptomatic Hypoglycemia after Metabolic Surgery: New Insights from Perioperative Continuous Glucose Monitoring
- Medical Nutrition Therapy Using Continuous Glucose Monitoring System