Acute Crit Care.
2022 May;37(2):177-184. 10.4266/acc.2021.01312.
The role of nafamostat mesilate as a regional anticoagulant during extracorporeal membrane oxygenation
- Affiliations
-
- 1Division of Pulmonology, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 2Division of Pulmonology and Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Anesthesiology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 4Department of Thoracic and Cardiovascular Surgery, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 5Department of Clinical Pharmacology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea
- 6Department of Internal Medicine, Kyung Hee University Medical Center, Kyung Hee University College of Medicine, Seoul, Korea
- 7Department of General Surgery, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- KMID: 2531674
- DOI: http://doi.org/10.4266/acc.2021.01312
Abstract
- Background
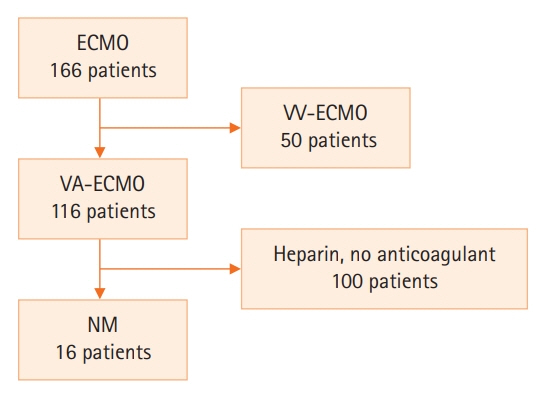
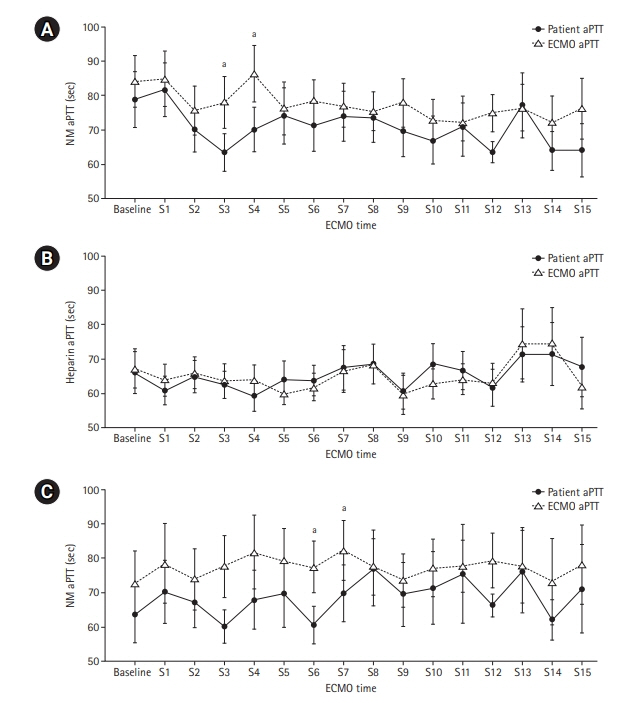
Anticoagulation during extracorporeal membrane oxygenation (ECMO) usually is required to prevent thrombosis. The aim of this study was to investigate the usefulness of nafamostat mesilate (NM) as a regional anticoagulant during veno-arterial ECMO (VA-ECMO) treatment. Methods: We retrospectively reviewed the medical records of 16 patients receiving VA-ECMO and NM from January 2017 to June 2020 at Haeundae Paik Hospital. We compared clinical and laboratory data, including activated partial thromboplastin time (aPTT), which was measured simultaneously in patients and the ECMO site, to estimate the efficacy of regional anticoagulation. Results: The median patient age was 68.5 years, and 56.3% of patients were men. Cardiovascular disease was the most common primary disease (75.0%) requiring ECMO treatment, followed by respiratory disease (12.5%). The median duration of ECMO treatment was 7.5 days. Among 16 patients, seven were switched to NM after first using heparin as an anticoagulation agent, and nine received only NM. When comparing aPTT values in the NM group between patients and the ECMO site, that in patients was significantly lower than that at the ECMO site (73.57 vs. 79.25 seconds; P=0.010); in contrast, no difference was observed in the heparin group. Conclusions: NM showed efficacy as a regional anticoagulation method by sustaining a lower aPTT value compared to that measured at the ECMO site. NM should be considered as a safer regional anticoagulation method in VA-ECMO for patients at high risk of bleeding.
Keyword
Figure
Reference
-
1. Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017; 63:60–7.
Article2. Buchtele N, Staudinger T, Schäfer AK, Bögl MS, Schoergenhofer C, Schwameis M. Anticoagulation in critically ill adults during extracorporeal circulation. Hamostaseologie. 2021; 41:294–306.
Article3. Esper SA, Levy JH, Waters JH, Welsby IJ. Extracorporeal membrane oxygenation in the adult: a review of anticoagulation monitoring and transfusion. Anesth Analg. 2014; 118:731–43.4. Olson SR, Murphree CR, Zonies D, Meyer AD, Mccarty OJ, Deloughery TG, et al. Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J. 2021; 67:290–6.
Article5. Protti A, Iapichino GE, Di Nardo M, Panigada M, Gattinoni L. Anticoagulation management and antithrombin supplementation practice during veno-venous extracorporeal membrane oxygenation: a worldwide survey. Anesthesiology. 2020; 132:562–70.6. Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev. 2015; 29:90–101.
Article7. Selleng S, Selleng K. Heparin-induced thrombocytopenia in cardiac surgery and critically ill patients. Thromb Haemost. 2016; 116:843–51.
Article8. Akizawa T, Koshikawa S, Ota K, Kazama M, Mimura N, Hirasawa Y. Nafamostat mesilate: a regional anticoagulant for hemodialysis in patients at high risk for bleeding. Nephron. 1993; 64:376–81.
Article9. Sadahiro T, Yuzawa H, Kimura T, Oguchi M, Morito T, Mizushima S, et al. Current practices in acute blood purification therapy in Japan and topics for further study. Contrib Nephrol. 2018; 196:209–14.
Article10. Makino S, Egi M, Kita H, Miyatake Y, Kubota K, Mizobuchi S. Comparison of nafamostat mesilate and unfractionated heparin as anticoagulants during continuous renal replacement therapy. Int J Artif Organs. 2016; 39:16–21.
Article11. Choi JY, Kang YJ, Jang HM, Jung HY, Cho JH, Park SH, et al. Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk: a randomized clinical trial. Medicine (Baltimore). 2015; 94:e2392.12. Kumar G, Maskey A. Anticoagulation in ECMO patients: an overview. Indian J Thorac Cardiovasc Surg. 2021; 37(Suppl 2):241–7.
Article13. Buscher H, Vukomanovic A, Benzimra M, Okada K, Nair P. Blood and anticoagulation management in extracorporeal membrane oxygenation for surgical and nonsurgical patients: a single-center retrospective review. J Cardiothorac Vasc Anesth. 2017; 31:869–75.
Article14. Cho HJ, Kim DW, Kim GS, Jeong IS. Anticoagulation therapy during extracorporeal membrane oxygenator support in pediatric patients. Chonnam Med J. 2017; 53:110–7.
Article15. Raiten JM, Wong ZZ, Spelde A, Littlejohn JE, Augoustides JG, Gutsche JT. Anticoagulation and transfusion therapy in patients requiring extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2017; 31:1051–9.
Article16. Muntean W. Coagulation and anticoagulation in extracorporeal membrane oxygenation. Artif Organs. 1999; 23:979–83.
Article17. Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth. 2009; 13:154–75.
Article18. Aoyama T, Ino Y, Ozeki M, Oda M, Sato T, Koshiyama Y, et al. Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments. Jpn J Pharmacol. 1984; 35:203–27.
Article19. Hwang SD, Hyun YK, Moon SJ, Lee SC, Yoon SY. Nafamostat mesilate for anticoagulation in continuous renal replacement therapy. Int J Artif Organs. 2013; 36:208–16.
Article20. Uchiba M, Okajima K, Abe H, Okabe H, Takatsuki K. Effect of nafamostat mesilate, a synthetic protease inhibitor, on tissue factor-factor VIIa complex activity. Thromb Res. 1994; 74:155–61.
Article21. Hitomi Y, Ikari N, Fujii S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Haemostasis. 1985; 15:164–8.
Article22. Baek NN, Jang HR, Huh W, Kim YG, Kim DJ, Oh HY, et al. The role of nafamostat mesylate in continuous renal replacement therapy among patients at high risk of bleeding. Ren Fail. 2012; 34:279–85.
Article23. Maruyama Y, Yoshida H, Uchino S, Yokoyama K, Yamamoto H, Takinami M, et al. Nafamostat mesilate as an anticoagulant during continuous veno-venous hemodialysis: a three-year retrospective cohort study. Int J Artif Organs. 2011; 34:571–6.
Article24. Han W, San Bok J, Cho HJ, Yu JH, Na MH, Kang S, et al. Single-center experience of extracorporeal membrane oxygenation mainly anticoagulated with nafamostat mesilate. J Thorac Dis. 2019; 11:2861–7.
Article25. Park JH, Her C, Min HK, Kim DK, Park SH, Jang HJ. Nafamostat mesilate as a regional anticoagulant in patients with bleeding complications during extracorporeal membrane oxygenation. Int J Artif Organs. 2015; 38:595–9.
Article26. Kikuchi M, Endo S, Inada K, Yamashita H, Takakuwa T, Nakae H, et al. Inhibitory effect of FUT-175 on the production of interleukin 8 and polymorphonuclear leukocyte elastase. Res Commun Mol Pathol Pharmacol. 1995; 87:269–74.27. Yoshikawa T, Murakami M, Furukawa Y, Kato H, Takemura S, Kondo M. Effects of FUT-175, a new synthetic protease inhibitor on endotoxin-induced disseminated intravascular coagulation in rats. Haemostasis. 1983; 13:374–8.
Article28. Kamijo H, Mochizuki K, Nakamura Y, Mori K, Ichikawa M, Nitta K, et al. Nafamostat mesylate improved survival outcomes of sepsis patients who underwent blood purification: a nationwide registry study in Japan. J Clin Med. 2020; 9:2629.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case involving the use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation in acute myocardial infarction
- Use of Nafamostat Mesilate as an Anticoagulant during Extracorporeal Membrane Oxygenation
- Validation of Nafamostat Mesilate as an Anticoagulant in Extracorporeal Membrane Oxygenation: A Large-Animal Experiment
- Prolonged Extracorporeal Lung Heart Assist ( Extracorporeal Membrane Oxygenation ) - 4 cases report
- Cardiac arrest caused by nafamostat mesilate