Endocrinol Metab.
2022 Jun;37(3):533-546. 10.3803/EnM.2022.1431.
Fancd2os Reduces Testosterone Production by Inhibiting Steroidogenic Enzymes and Promoting Cellular Apoptosis in Murine Testicular Leydig Cells
- Affiliations
-
- 1Department of Biochemistry and Molecular Biology, Basic Medical Science Center, School of Basic Medicine, Shanxi Medical University, Jinzhong, China
- KMID: 2531397
- DOI: http://doi.org/10.3803/EnM.2022.1431
Abstract
- Background
It is well-established that serum testosterone in men decreases with age, yet the underlying mechanism of this change remains elusive.
Methods
The expression patterns of Fancd2 opposite-strand (Fancd2os) in BALB/c male mice and testicular tissue derived cell lines (GC-1, GC-2, TM3, and TM4) were assessed using real-time polymerase chain reaction (RT-PCR), Western blot and immunofluorescence. The Fancd2os-overexpressing or knockdown TM3 cells were constructed by infecting them with lentivirus particles and were used to evaluated the function of Fancd2os. The testosterone production was measured using enzyme linked immunosorbent assay (ELISA) and the steroidogenic enzymes such as steroidogenic acute regulatory protein (StAR), P450 cholesterol side-chain cleavage (P450scc), and 3β-hydroxysteroid dehydrogenase (3β-HSD) were analysed using RT-PCR. The apoptosis of TM3 cells induced by ultraviolet light or testicular tissues was detected using flow cytometry, Western blot or dUTP-biotin nick end labeling (TUNEL) assays. Pearson correlation analysis was used to assess the correlation between the Fancd2os expression and TUNEL-positive staining in mouse testicular Leydig cells.
Results
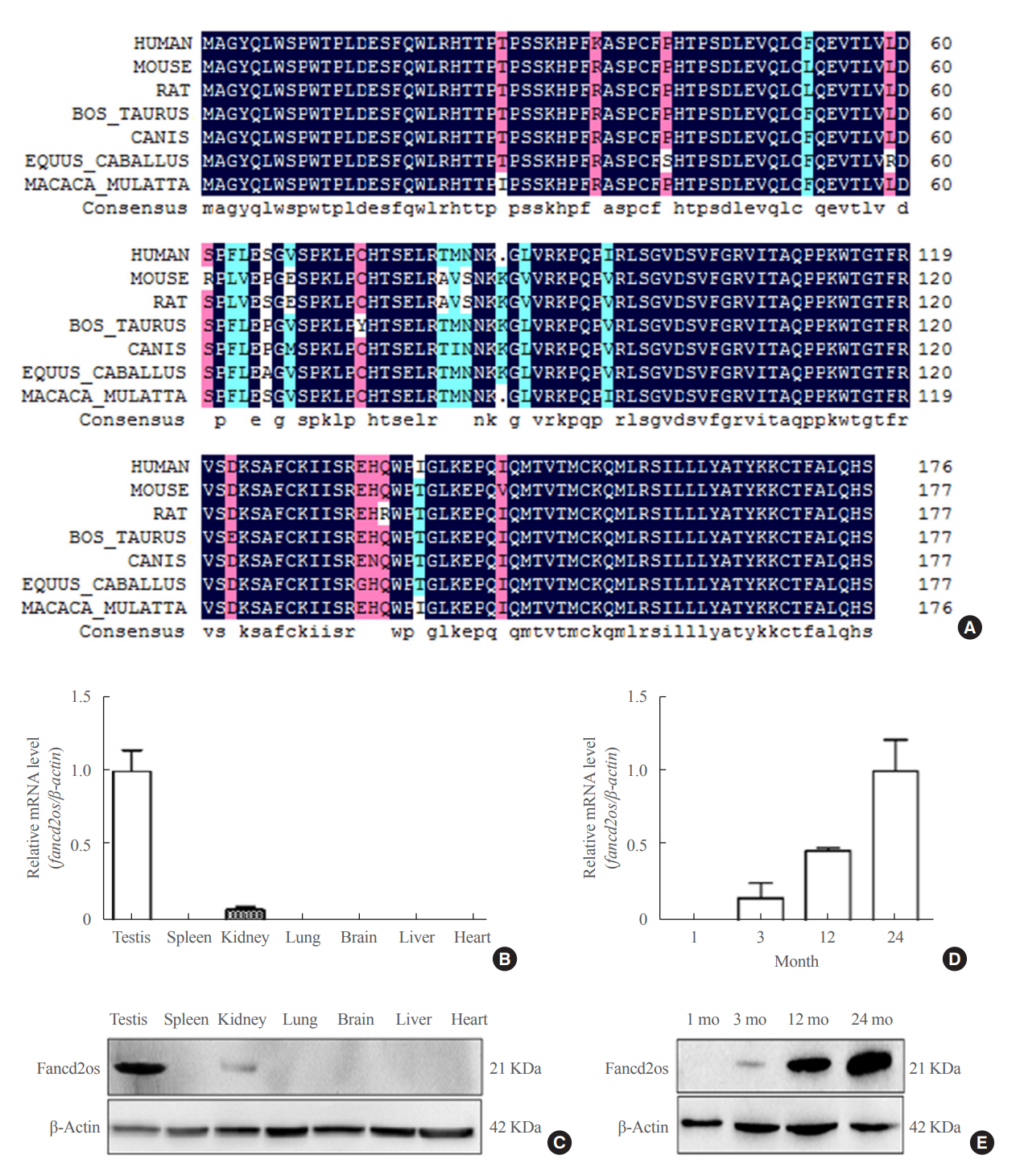
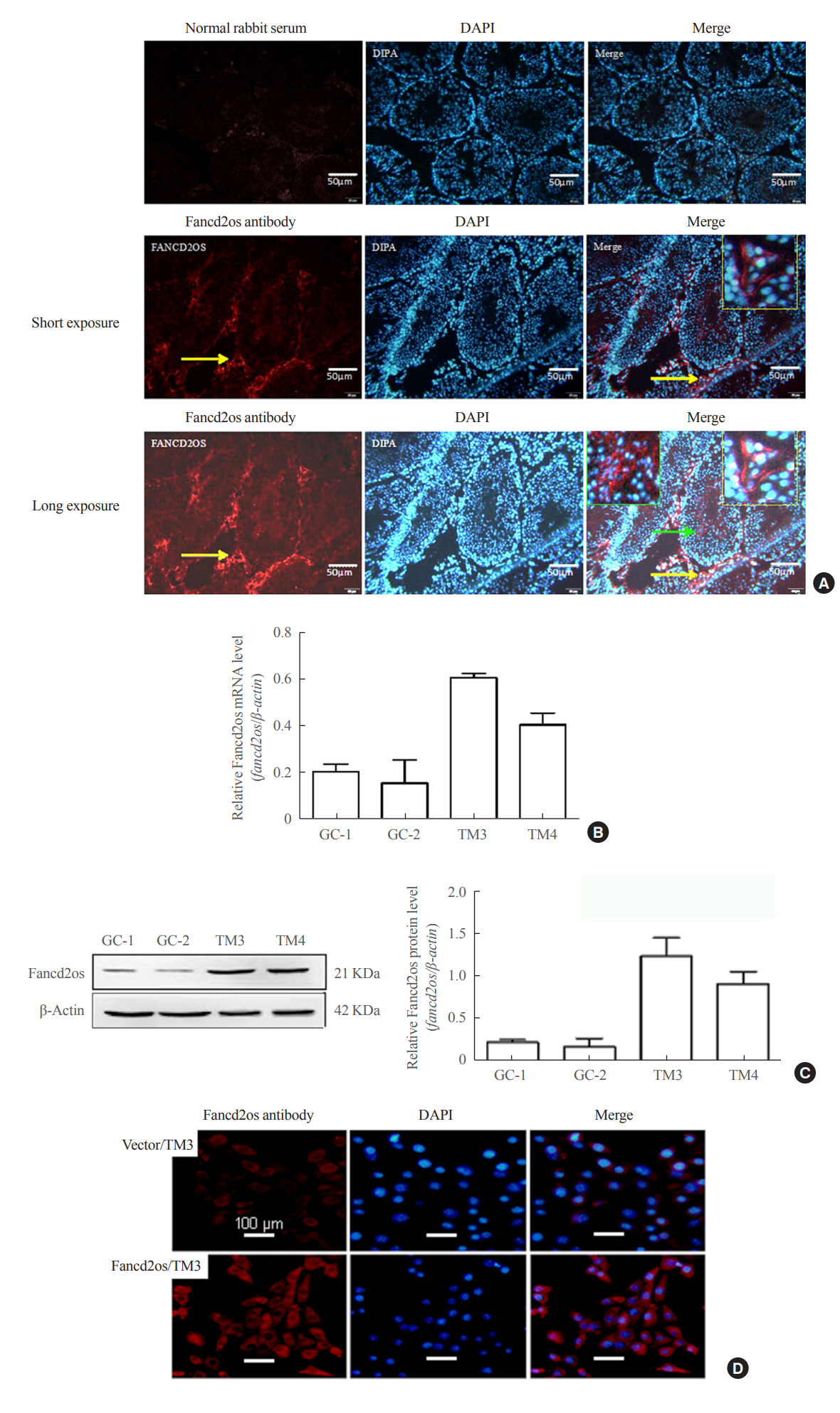
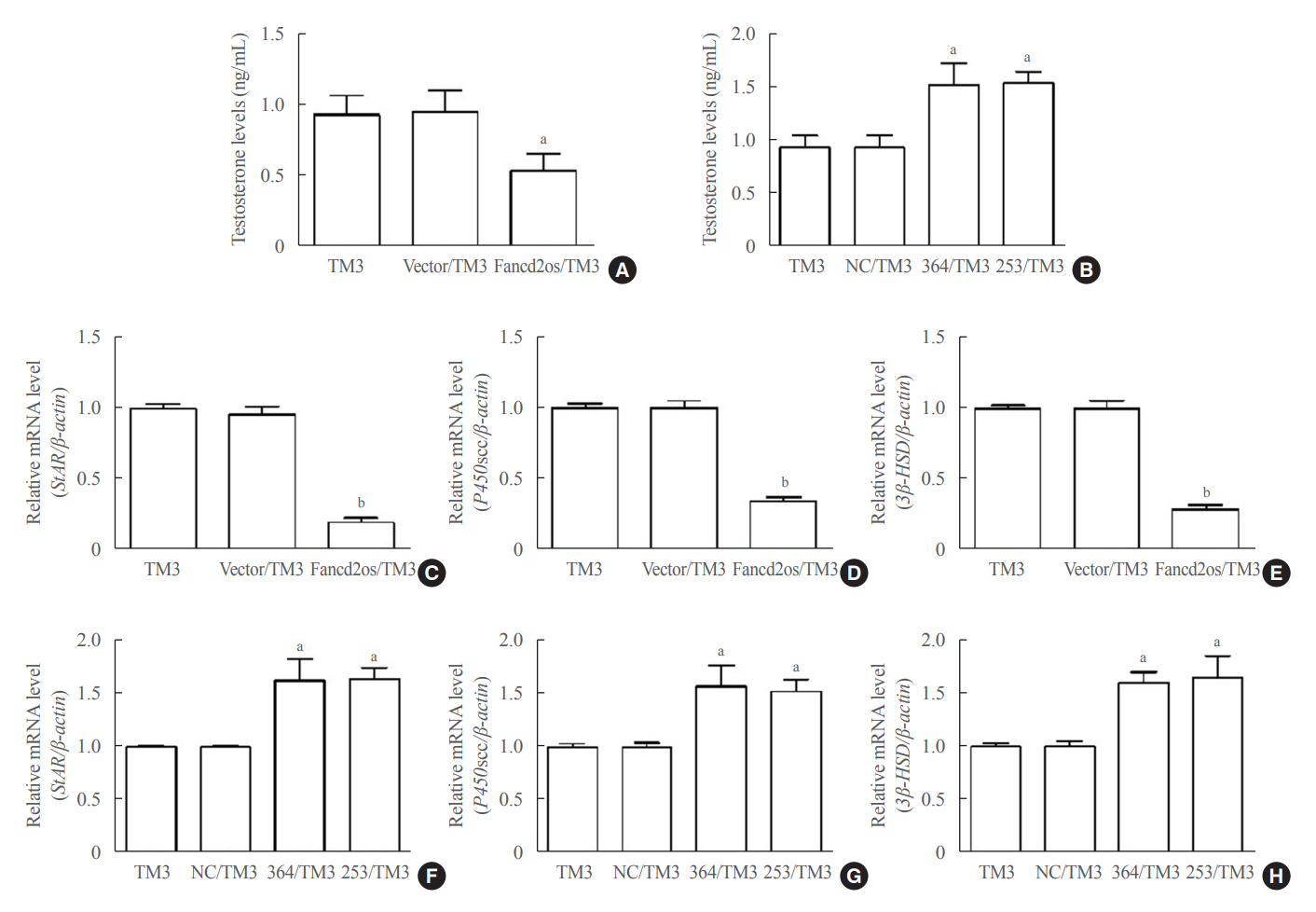
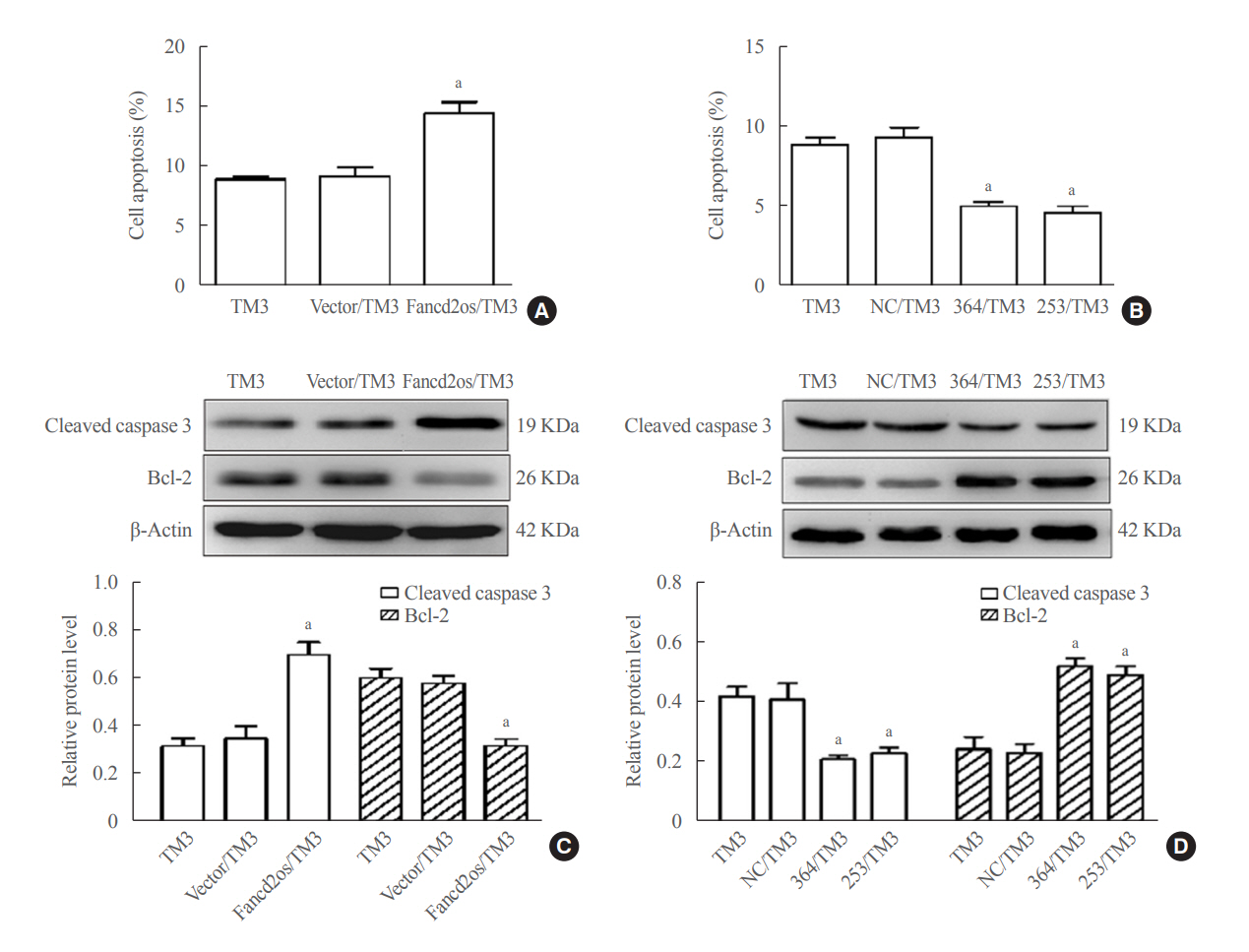
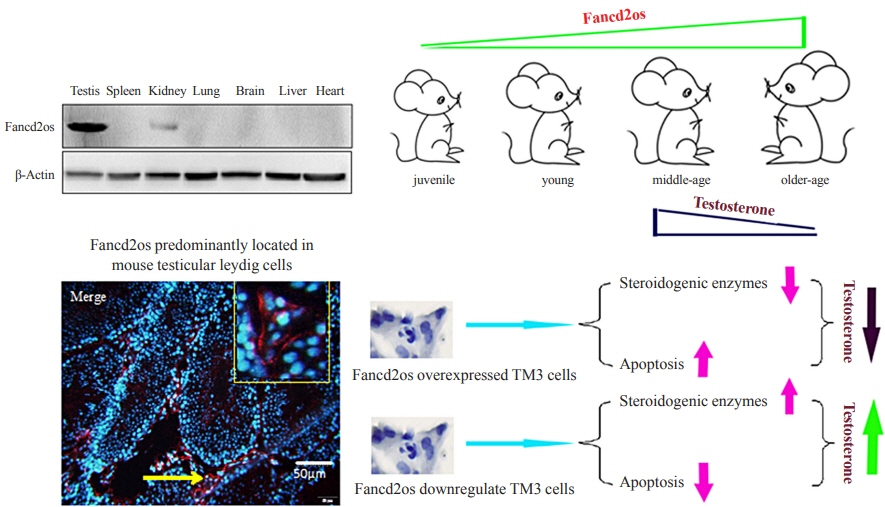
The Fancd2os protein was predominantly expressed in mouse testicular Leydig cells and its expression increased with age. Fancd2os overexpression inhibited testosterone levels in TM3 Leydig cells, whereas knockdown of Fancd2os elevated testosterone production. Fancd2os overexpression downregulated the levels of StAR, P450scc and 3β-HSD, while Fancd2os knockdown reversed this effect. Fancd2os overexpression promoted ultraviolet light-induced apoptosis of TM3 cells. In contrast, Fancd2os knockdown restrained apoptosis in TM3 cells. In vivo assays revealed that higher Fancd2os levels and mouse age were associated with increased apoptosis in Leydig cells and decreased serum testosterone levels. Pearson correlation analysis exhibited a strong positive correlation between the expression of Fancd2os and TUNEL-positive staining in mouse testicular Leydig cells.
Conclusion
Our findings suggest that Fancd2os regulates testosterone synthesis via both steroidogenic enzymes and the apoptotic pathway.
Figure
Reference
-
1. Chen H, Ge RS, Zirkin BR. Leydig cells: from stem cells to aging. Mol Cell Endocrinol. 2009; 306:9–16.
Article2. Sun D, Dong W, Jin B, Chen G, Cai B, Deng W, et al. Mechanisms of Yangjing capsule in Leydig cell apoptosis and testosterone synthesis via promoting StAR expression. Biol Pharm Bull. 2018; 41:1401–5.3. Wang X, Zou Z, Yang Z, Jiang S, Lu Y, Wang D, et al. HIF 1 inhibits StAR transcription and testosterone synthesis in murine Leydig cells. J Mol Endocrinol. 2019; 62:1–13.
Article4. Xu A, Li X, Li K, Zhang J, Li Y, Gong D, et al. Linoleic acid promotes testosterone production by activating Leydig cell GPR120/ERK pathway and restores BPA-impaired testicular toxicity. Steroids. 2020; 163:108677.5. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002; 87:589–98.
Article6. Colasante A, Minasi MG, Scarselli F, Casciani V, Zazzaro V, Ruberti A, et al. The aging male: relationship between male age, sperm quality and sperm DNA damage in an unselected population of 3124 men attending the fertility centre for the first time. Arch Ital Urol Androl. 2019; 90:254–9.
Article7. Cohen J, Nassau DE, Patel P, Ramasamy R. Low testosterone in adolescents & young adults. Front Endocrinol (Lausanne). 2020; 10:916.
Article8. Swerdloff RS, Wang C, Bhasin S. Developments in the control of testicular function. Baillieres Clin Endocrinol Metab. 1992; 6:451–83.9. Lee J, Tong T, Duan H, Foong YH, Musaitif I, Yamazaki T, et al. Regulation of StAR by the N-terminal domain and coinduction of SIK1 and TIS11b/Znf36l1 in single cells. Front Endocrinol (Lausanne). 2016; 7:107.
Article10. Kumar S, Kim HJ, Lee CH, Choi HS, Lee K. Leydig cell-specific DAX1-deleted mice has higher testosterone level in the testis during pubertal development. Reprod Sci. 2022; 29:955–62.
Article11. Henriksen K, Hakovirta H, Parvinen M. Testosterone inhibits and induces apoptosis in rat seminiferous tubules in a stage-specific manner: in situ quantification in squash preparations after administration of ethane dimethane sulfonate. Endocrinology. 1995; 136:3285–91.
Article12. Andersen CY, Byskov AG, Grinsted J. Growth pattern of the sex ducts in foetal mouse hermaphrodites. J Embryol Exp Morphol. 1983; 73:59–68.
Article13. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999; 15:269–90.
Article14. Cohen JJ. Apoptosis. Immunol Today. 1993; 14:126–30.
Article15. Aragane Y, Kulms D, Metze D, Wilkes G, Poppelmann B, Luger TA, et al. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998; 140:171–82.
Article16. Teraki Y, Shiohara T. Apoptosis and the skin. Eur J Dermatol. 1999; 9:413–25.17. Kwon JT, Jin S, Choi H, Kim J, Jeong J, Kim J, et al. Identification and characterization of germ cell genes expressed in the F9 testicular teratoma stem cell line. PLoS One. 2014; 9:e103837.
Article18. Wang X, Pan L, Zou Z, Wang D, Lu Y, Dong Z, et al. Hypoxia reduces testosterone synthesis in mouse Leydig cells by inhibiting NRF1-activated StAR expression. Oncotarget. 2017; 8:16401–13.
Article19. Zhou X, Wang WT, Sun J, Liu HY, Bai XY, Liu JJ, et al. ANKRD49 inhibits etoposide-induced intrinsic apoptosis of GC-1 cells by modulating NF-κB signaling. Mol Cell Biochem. 2019; 457:21–9.
Article20. Hassan EA, Hassan MH, Torad FA. Correlation between clinical severity and type and degree of pectus excavatum in twelve brachycephalic dogs. J Vet Med Sci. 2018; 80:766–71.
Article21. Tsuji-Hosokawa A, Kashimada K, Kato T, Ogawa Y, Nomura R, Takasawa K, et al. Peptidyl arginine deiminase 2 (Padi2) is expressed in Sertoli cells in a specific manner and regulated by SOX9 during testicular development. Sci Rep. 2018; 8:13263.
Article22. Zhang Y, Ge R, Hardy MP. Androgen-forming stem Leydig cells: identification, function and therapeutic potential. Dis Markers. 2008; 24:277–86.
Article23. Rebourcet D, Mackay R, Darbey A, Curley MK, Jorgensen A, Frederiksen H, et al. Ablation of the canonical testosterone production pathway via knockout of the steroidogenic enzyme HSD17B3, reveals a novel mechanism of testicular testosterone production. FASEB J. 2020; 34:10373–86.
Article24. Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008; 295:115–20.
Article25. Othman AI, Edrees GM, El-Missiry MA, Ali DA, Aboel-Nour M, Dabdoub BR. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol Ind Health. 2016; 32:1537–49.
Article26. Stergiou L, Eberhard R, Doukoumetzidis K, Hengartner MO. NER and HR pathways act sequentially to promote UV-C-induced germ cell apoptosis in Caenorhabditis elegans. Cell Death Differ. 2011; 18:897–906.
Article27. Nadzialek S, Pigneur LM, Weron B, Kestemont P. Bcl-2 and caspase 3 mRNA levels in the testes of gudgeon, Gobio gobio, exposed to ethinylestradiol (EE2). Aquat Toxicol. 2010; 98:304–10.
Article28. Saad F, Rohrig G, von Haehling S, Traish A. Testosterone deficiency and testosterone treatment in older men. Gerontology. 2017; 63:144–56.
Article29. Abai B. StatPearls. Treasure Island: StatPearls Publishing;2022. Chapter, Physiology, male reproductive system [cited 2022 Jun 8]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538429.30. Chung HJ, Noh Y, Kim MS, Jang A, Lee CE, Myung SC. Steroidogenic effects of Taraxacum officinale extract on the levels of steroidogenic enzymes in mouse Leydig cells. Anim Cells Syst (Seoul). 2018; 22:407–14.31. Wu CY, Yu TJ, Chen MJ. Age related testosterone level changes and male andropause syndrome. Chang Gung Med J. 2000; 23:348–53.32. Krause W. Androgens in the demography of male life course: a review. Soc Biol. 2006; 53:4–12.33. Grainger SA, Mead JK, Vanman EJ, Henry JD. The relationship between testosterone and social cognition in younger and older adults. Biol Psychol. 2021; 161:108072.
Article34. Machida T, Yonezawa Y, Noumura T. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm Behav. 1981; 15:238–45.
Article35. Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells: characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem. 1994; 269:28314–22.
Article36. Ye L, Su ZJ, Ge RS. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules. 2011; 16:9983–10001.
Article37. Helal MA, Mehmet H, Thomas NS, Cox PM, Ralph DJ, Bajoria R, et al. Ontogeny of human fetal testicular apoptosis during first, second, and third trimesters of pregnancy. J Clin Endocrinol Metab. 2002; 87:1189–93.
Article38. O’Hara L, McInnes K, Simitsidellis I, Morgan S, Atanassova N, Slowikowska-Hilczer J, et al. Autocrine androgen action is essential for Leydig cell maturation and function, and protects against late-onset Leydig cell apoptosis in both mice and men. FASEB J. 2015; 29:894–910.
Article39. Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004; 233:181–241.
Article40. Zheng Y, Lei Q, Jongejan A, Mulder CL, van Daalen SK, Mastenbroek S, et al. The influence of retinoic acid-induced differentiation on the radiation response of male germline stem cells. DNA Repair (Amst). 2018; 70:55–66.
Article41. Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013; 100:1180–6.
Article42. Pustisek N, Situm M. UV-radiation, apoptosis and skin. Coll Antropol. 2011; 35 Suppl 2:339–41.43. Dai YL, Yang D, Song LH, Yang HM, Yu JB, Zheng F, et al. Low molecular weight oligosaccharide from Panax ginseng C.A. Meyer against UV-mediated apoptosis and inhibits tyrosinase activity in vitro and in vivo. Evid Based Complement Alternat Med. 2021; 2021:8879836.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- STEROIDOGENESIS IN THE TESTIS
- C-Type Natriuretic Peptide/Natriuretic Peptide Receptor 2 Is Involved in Cell Proliferation and Testosterone Production in Mouse Leydig Cells
- Korean Ginseng Berry Extract Enhances the Male Steroidogenesis Enzymes In Vitro and In Vivo
- An Experimental Study on the Role of Leydig Cell in Testicular Descent
- Effects of Recombinant Human Growth Hormone on the Onset of Puberty, Leydig Cell Differentiation, Spermatogenesis and Hypothalamic KISS1 Expression in Immature Male Rats