J Korean Diabetes.
2021 Mar;22(1):60-70. 10.4093/jkd.2021.22.1.60.
Effects of Jerusalem Artichoke Extract and Inulin on Blood Glucose Levels and Insulin Secretion in Streptozotocin Induced Diabetic Mice
- Affiliations
-
- 1Department of Internal Medicine, St. Carollo Hospital, Suncheon, Korea
- 2Department of Clinical Pathology, St. Carollo Hospital, Suncheon, Korea
- 3Department of Food and Nutrition, Sunchon National University, Suncheon, Korea
- 4Department of Pharmacy, Sunchon National University, Suncheon, Korea
- KMID: 2526182
- DOI: http://doi.org/10.4093/jkd.2021.22.1.60
Abstract
- Background
To determine the effects of Jerusalem Artichoke extract (JAE) and inulin on blood glucose levels and insulin secretion in streptozotocin (STZ)-induced diabetic mice.
Methods
Thirty four mice were divided into a normal control group and three experimental groups: diabetic control, JAE, and inulin. STZ (50 mg/kg) was injected intraperitoneally to induce diabetes in the three experimental groups. The JAE and inulin groups were fed 10 g/kg JAE or fed 1 g/kg inulin, respectively, for 6 weeks. Fasting glucose was checked weekly. After 6 weeks, the oral glucose tolerance test (OGTT) was performed, and the insulin level was checked.
Results
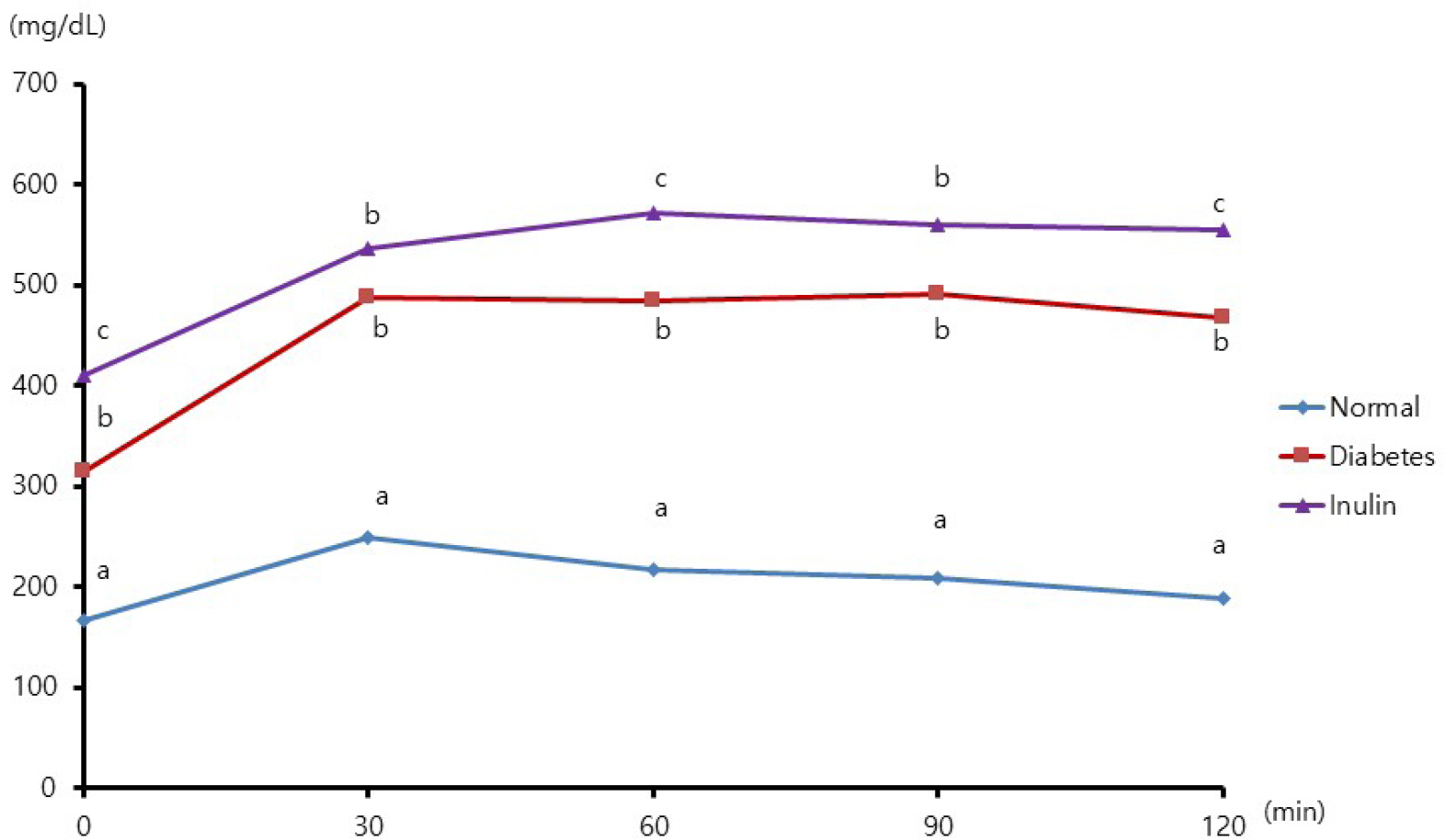
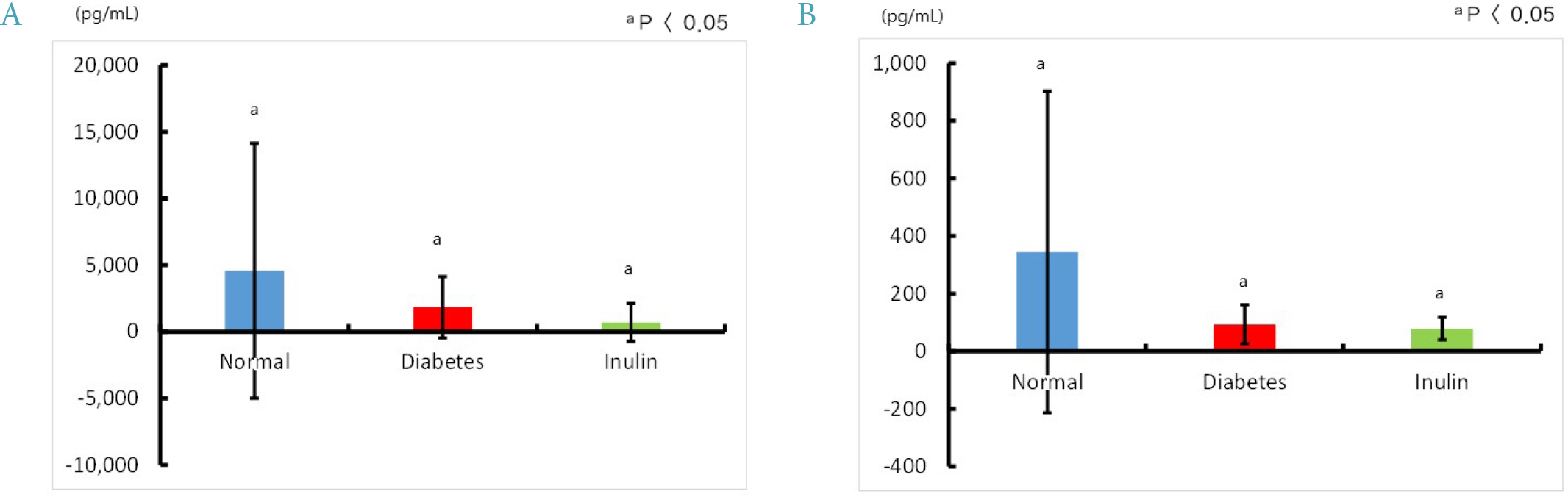
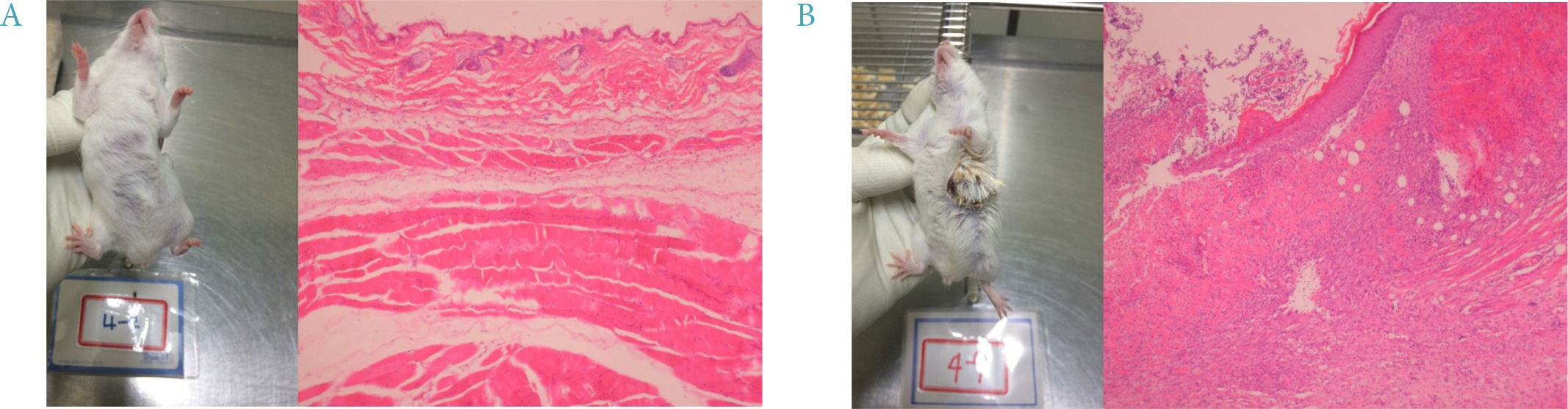
Four mice from the JAE group (n = 9) died and autopsies revealed inflammation and ulceration of skin lesions on the chest areas. Fasting glucose levels were not decreased in the inulin or JAE group relative to diabetic control group. In the OGTT at 60 minutes and 120 minutes, the serum glucose levels were significantly higher in the inulin group (572.6 ± 52.0 mg/dL and 555.8 ± 72.9 mg/dL, respectively) than in diabetic control group (484.3 ± 81.6 mg/dL and 467.3 ± 111.1 mg/dL, respectively). Insulin levels were not increased in the inulin group relative to the diabetic control group.
Conclusion
These results indicate that JAE and inulin might not be useful therapeutic strategies for diabetes mellitus and indiscreet intake of Jerusalem Artichoke could exacerbate to diabetes.
Keyword
Figure
Reference
-
1. Lee KW. Costs of diabetes mellitus in Korea. Diabetes Metab J. 2011. 35:567–70.2. Kim JH., Kim DJ., Jang HC., Choi SH. Epidemiology of micro- and macrovascular complications of type 2 diabetes in Korea. Diabetes Metab J. 2011. 35:571–7.3. Gaede P., Lund-Andersen H., Parving HH., Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008. 358:580–91.4. Kim BY., Won JC., Lee JH., Kim HS., Park JH., Ha KH, et al. Diabetes fact sheets in Korea, 2018: an appraisal of current status. Diabetes Metab J. 2019. 43:487–94.5. Kim SG., Choi DS. The present state of diabetes mellitus in Korea. J Korean Med Assoc. 2008. 51:791–8.6. Korean Diabetes Association. Diabetes in Korea 2007. Report of Task Force Team for basic statistical study of Korean diabetes mellitus. Seoul: Korean Diabetes Association;2007.7. Egede LE., Ye X., Zheng D., Silverstein MD. The prevalence and pattern of complementary and alternative medicine use in individuals with diabetes. Diabetes Care. 2002. 25:324–9.8. Cho MR., Choue R. A study of folk remedies in type II diabetic patients. Korean J Nutr. 1998. 31:1151–7.9. Roberfroid M. Functional food concept and its application to prebiotics. Dig Liver Dis. 2002. 34(Suppl 2):S105–10.10. Kim JL., Bae CR., Cha YS. Helianthus tuberosus extract has anti-diabetes effects in HIT-T15 cells. J Korean Soc Food Sci Nutr. 2010. 39:31–5.11. Luo J., Rizkalla SW., Alamowitch C., Boussairi A., Blayo A., Barry JL, et al. Chronic consumption of short-chain fructooligosaccharides by healthy subjects decreased basal hepatic glucose production but had no effect on insulin-stimulated glucose metabolism. Am J Clin Nutr. 1996. 63:939–45.12. Bonsu NK., Johnson CS., McLeod KM. Can dietary fructans lower serum glucose? J Diabetes. 2011. 3:58–66.13. Nathan DM., Buse JB., Davidson MB., Heine RJ., Holman RR., Sherwin R, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006. 29:1963–72.14. Kaur N., Gupta AK. Applications of inulin and oligofructose in health and nutrition. J Biosci. 2002. 27:703–14.15. Wang Z., Hwang SH., Lee SY., Lim SS. Fermentation of purple Jerusalem artichoke extract to improve the α-glucosidase inhibitory effect in vitro and ameliorate blood glucose in db/db mice. Nutr Res Pract. 2016. 10:282–7.16. Dehghan P., Pourghassem Gargari B., Asghari Jafarabadi M. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized controlled clinical trial. Nutrition. 2014. 30:418–23.17. Kim HJ., Kim DI., Yon JM. Effects of Jerusalem artichoke (Helianthus tuberosus L.) extracts on blood glucose and lipid metabolism in STZ-induced diabetic rats. Korean J Clin Lab Sci. 2015. 47:203–8.18. Yang HJ., Kwon DY., Kim MJ., Kang S., Kim DS., Park S. Jerusalem artichoke and chungkookjang additively improve insulin secretion and sensitivity in diabetic rats. Nutr Metab (Lond). 2012. 9:112.19. Meshcheriakova VA., Plotnikova OA., Sharafetdinov KhKh., Iatsyshina TA. Use of Jerusalem artichokes in diet therapy of patients with type II diabetes mellitus. Vopr Pitan. 1995. (3):24–7. Russian.20. Evert AB., Boucher JL., Cypress M., Dunbar SA., Franz MJ., Mayer-Davis EJ, et al. American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013. 36:3821–42.21. Kim HN., Yu SY., Yoon WB., Jang SM., Jang YJ., Lee OH. Analysis of nutritional components and physicochemical properties of hot-air dried Jerusalem artichoke (Helianthus tuberosus L.) powder. Korean J Food Sci Technol. 2014. 46:73–8.22. Jang HL., Hong JY., Kim NJ., Kim MH., Shin SR., Yoon KY. Comparison of nutrient components and physicochemical properties of general and colored potato. Korean J Hortic Sci Technol. 2011. 29:144–50.23. Dunn JD., Benton WW., Orozco-Torrentera E., Adamson RT. The burden of hyperkalemia in patients with cardiovascular and renal disease. Am J Manag Care. 2015. 21(15 Suppl):s307–15.24. Coppens P., da Silva MF., Pettman S. European regulations on nutraceuticals, dietary supplements and functional foods: a framework based on safety. Toxicology. 2006. 221:59–74.25. Paik DJ., Lee CH. Review of cases of patient risk associated with ginseng abuse and misuse. J Ginseng Res. 2015. 39:89–93.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Hyperglycemia Induced by Jerusalem Artichoke in a Patient with Type 2 Diabetes Mellitus
- Jerusalem Artichoke and Inulin
- Fermentation of purple Jerusalem artichoke extract to improve the α-glucosidase inhibitory effect in vitro and ameliorate blood glucose in db/db mice
- The Role of the Central Parasympathetic Nervous System in Modulating Glucose Metabolism in Streptozotocin-induced Diabetic Rats
- Effects of Polygonatum odoratum on In vivo Insulin Activity in Streptozotocin-Induced Diabetic Rats