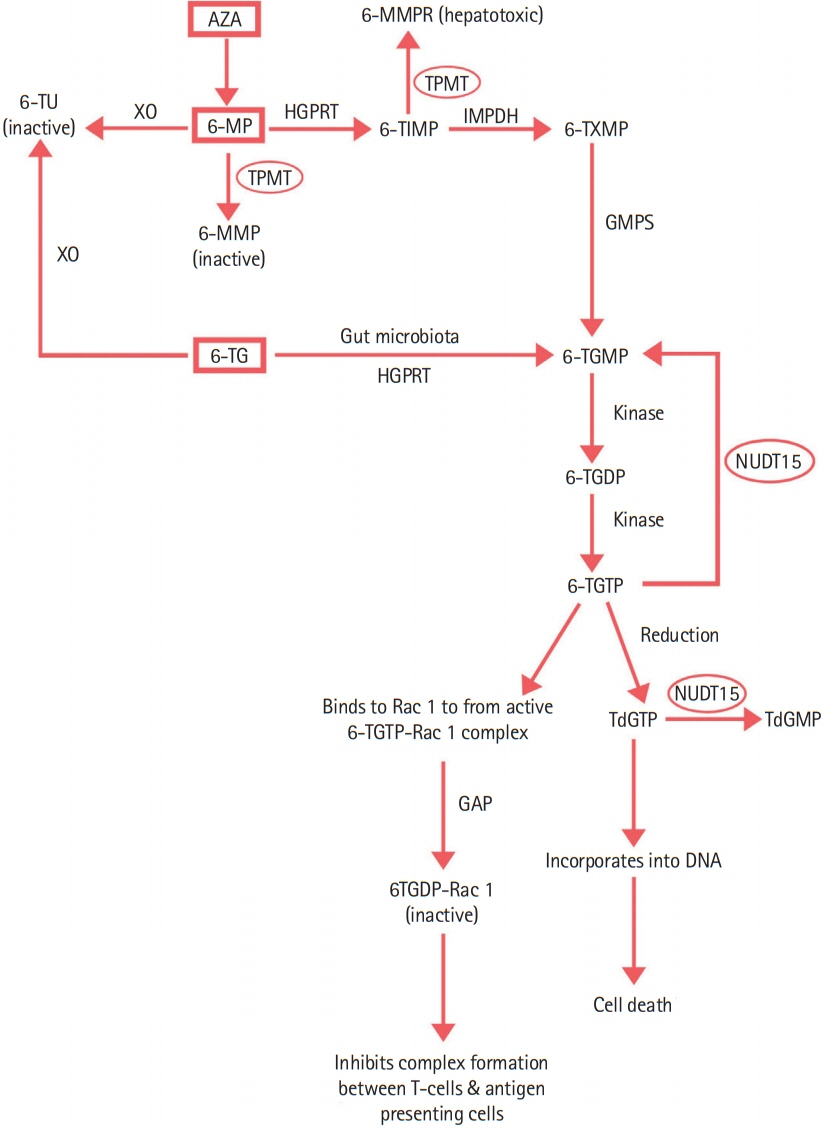
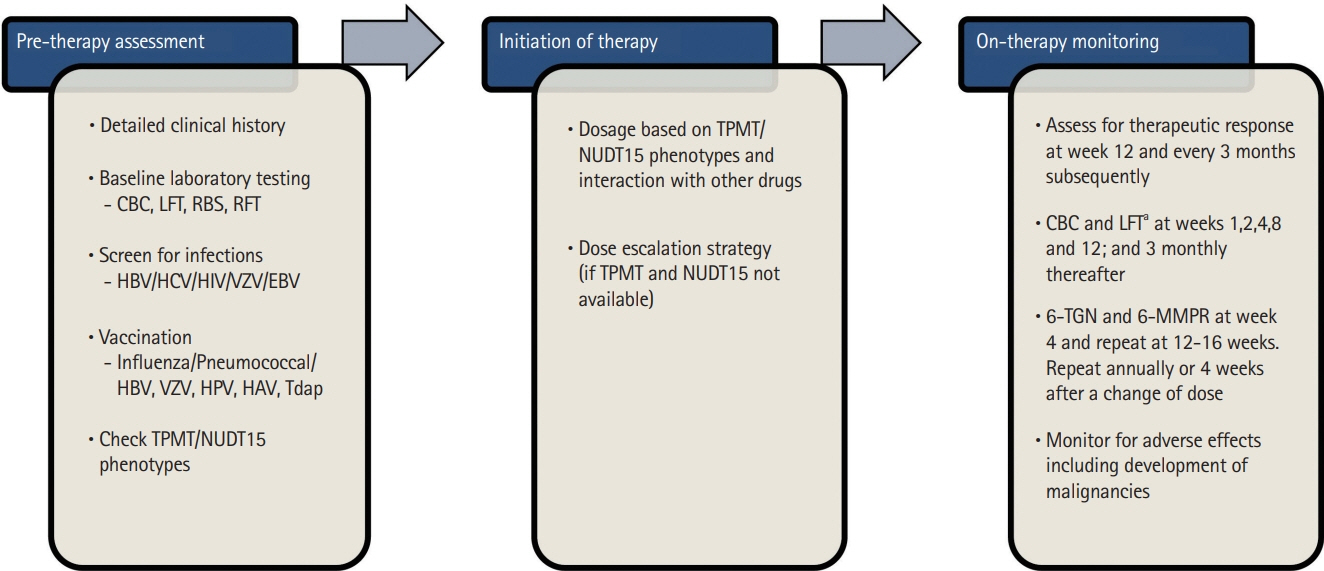
Intest Res.
2022 Jan;20(1):11-30. 10.5217/ir.2020.00155.
Use of thiopurines in inflammatory bowel disease: an update
- Affiliations
-
- 1Department of Gastroenterology, Dayanand Medical College and Hospital, Ludhiana, India
- 2Department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences, New Delhi, India
- 3Department of Gastroenterology, Christian Medical College, Vellore, India
- 4University of Manitoba IBD Clinical and Research Centre, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 5P. D. Hinduja Hospital and Medical Research Centre, Mumbai, India
- 6Department of Gastroenterology, Kasturba Medical College, Manipal, India
- 7Asian Institute of Gastroenterology Hyderabad, Hyderabad, India
- 8Department of Medicine and Therapeutics, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong
- 9Department of Pharmacology, Dayanand Medical College and Hospital, Ludhiana, India
- 10Citizens Centre for Digestive Disorders, Hyderabad, India
- 11Department of Gastroenterology, Helsinki University Central Hospital, Helsinki, Finland
- 12Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- 13Department of Medicine, Northwestern University, Chicago, IL, USA
- 14Department of Internal Medicine, Dayanand Medical College, Ludhiana, India
- KMID: 2525069
- DOI: http://doi.org/10.5217/ir.2020.00155
Abstract
- Inflammatory bowel disease (IBD), once considered a disease of the Western hemisphere, has emerged as a global disease. As the disease prevalence is on a steady rise, management of IBD has come under the spotlight. 5-Aminosalicylates, corticosteroids, immunosuppressive agents and biologics are the backbone of treatment of IBD. With the advent of biologics and small molecules, the need for surgery and hospitalization has decreased. However, economic viability and acceptability is an important determinant of local prescription patterns. Nearly one-third of the patients in West receive biologics as the first/initial therapy. The scenario is different in developing countries where biologics are used only in a small proportion of patients with IBD. Increased risk of reactivation of tuberculosis and high cost of the therapy are limitations to their use. Thiopurines hence become critical for optimal management of patients with IBD in these regions. However, approximately one-third of patients are intolerant or develop adverse effects with their use. This has led to suboptimal use of thiopurines in clinical practice. This review article discusses the clinical aspects of thiopurine use in patients with IBD with the aim of optimizing their use to full therapeutic potential.
Keyword
Figure
Cited by 3 articles
-
NUDT15 Genotyping in Thiopurine Drug Therapy
Jong Kwon Lee, Rihwa Choi, Soo-Youn Lee
Lab Med Online. 2022;12(4):217-226. doi: 10.47429/lmo.2022.12.4.217.Prevention of postoperative recurrence in Crohn’s disease: the never-ending story
Jung-Bin Park, Sang Hyoung Park
Intest Res. 2022;20(3):279-280. doi: 10.5217/ir.2022.00081.Infectious complications in patients with inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting
Yu Kyung Jun, Seong-Joon Koh, Dae Seong Myung, Sang Hyoung Park, Choon Jin Ooi, Ajit Sood, Jong Pil Im
Intest Res. 2023;21(3):353-362. doi: 10.5217/ir.2023.00013.
Reference
-
1. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020; 5:17–30.2. Qiu Y, Mao R, Zhang SH, et al. Safety profile of thiopurines in Crohn disease: analysis of 893 patient-years follow-up in a Southern China cohort. Medicine (Baltimore). 2015; 94:e1513.3. Eklund BI, Moberg M, Bergquist J, Mannervik B. Divergent activities of human glutathione transferases in the bioactivation of azathioprine. Mol Pharmacol. 2006; 70:747–754.
Article4. Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004; 2:731–743.
Article5. Chande N, Townsend CM, Parker CE, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2016; 10–CD000545.
Article6. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010; 362:1383–1395.
Article7. Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015; (10):CD000067.
Article8. Mantzaris GJ, Christidou A, Sfakianakis M, et al. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis. 2009; 15:375–382.
Article9. Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV Jr. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn’s disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015; 148:64–76.
Article10. Gjuladin-Hellon T, Iheozor-Ejiofor Z, Gordon M, Akobeng AK. Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn’s disease. Cochrane Database Syst Rev. 2019; 8–CD010233.
Article11. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology. 2017; 152:277–295.
Article12. Klein M, Binder HJ, Mitchell M, Aaronson R, Spiro H. Treatment of Crohn’s disease with azathioprine: a controlled evaluation. Gastroenterology. 1974; 66:916–922.
Article13. Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine: a long-term, randomized, double-blind study. N Engl J Med. 1980; 302:981–987.14. Steele SR, Kumar R, Feingold DL, Rafferty JL, Buie WD; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of perianal abscess and fistula-in-ano. Dis Colon Rectum. 2011; 54:1465–1474.
Article15. Ford AC, Luthra P, Hanauer SB, Travis SP, Harris MS, Reinisch W. Placebo response rate in clinical trials of fistulizing Crohn’s disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014; 12:1981–1990.
Article16. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020; 14:4–22.17. Lee MJ, Parker CE, Taylor SR, et al. Efficacy of medical therapies for fistulizing Crohn’s disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018; 16:1879–1892.
Article18. Sood A, Midha V, Sood N, Kaushal V. Role of azathioprine in severe ulcerative colitis: one-year, placebo-controlled, randomized trial. Indian J Gastroenterol. 2000; 19:14–16.19. Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006; 55:47–53.
Article20. Maté-Jiménez J, Hermida C, Cantero-Perona J, Moreno-Otero R. 6-Mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000; 12:1227–1233.
Article21. Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and metaanalysis. Am J Gastroenterol. 2011; 106:630–642.
Article22. Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014; 146:392–400.
Article23. Sood A, Kaushal V, Midha V, Bhatia KL, Sood N, Malhotra V. The beneficial effect of azathioprine on maintenance of remission in severe ulcerative colitis. J Gastroenterol. 2002; 37:270–274.
Article24. Timmer A, Patton PH, Chande N, McDonald JW, MacDonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016; 2016–CD000478.
Article25. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020; 158:1450–1461.
Article26. Park SK, Yang SK, Ye BD, et al. The long-term efficacy of azathioprine in steroid-dependent ulcerative colitis. Scand J Gastroenterol. 2013; 48:1386–1393.
Article27. Goel RM, Blaker P, Mentzer A, Fong SC, Marinaki AM, Sanderson JD. Optimizing the use of thiopurines in inflammatory bowel disease. Ther Adv Chronic Dis. 2015; 6:138–146.
Article28. Sood A, Midha V, Sood N, Bansal M. Long term results of use of azathioprine in patients with ulcerative colitis in India. World J Gastroenterol. 2006; 12:7332–7336.
Article29. Sood R, Ansari S, Clark T, Hamlin PJ, Ford AC. Long-term efficacy and safety of azathioprine in ulcerative colitis. J Crohns Colitis. 2015; 9:191–197.
Article30. Lennard L, Chew TS, Lilleyman JS. Human thiopurine methyltransferase activity varies with red blood cell age. Br J Clin Pharmacol. 2001; 52:539–546.
Article31. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017; 67:370–398.32. Viazis N, Vlachogiannakos J, Georgiou O, et al. Course of inflammatory bowel disease in patients infected with human immunodeficiency virus. Inflamm Bowel Dis. 2010; 16:507–511.
Article33. Gidrewicz D, Lehman D, Rabizadeh S, Majlessipour F, Dubinsky M. Primary EBV infection resulting in lymphoproliferative disease in a teenager with Crohn disease. J Pediatr Gastroenterol Nutr. 2011; 52:103–105.
Article34. N’guyen Y, Andreoletti L, Patey M, et al. Fatal Epstein-Barr virus primo infection in a 25-year-old man treated with azathioprine for Crohn’s disease. J Clin Microbiol. 2009; 47:1252–1254.
Article35. Lam GY, Halloran BP, Peters AC, Fedorak RN. Lymphoproliferative disorders in inflammatory bowel disease patients on immunosuppression: lessons from other inflammatory disorders. World J Gastrointest Pathophysiol. 2015; 6:181–192.
Article36. Collie-Duguid ES, Pritchard SC, Powrie RH, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999; 9:37–42.
Article37. Kim JH, Cheon JH, Hong SS, et al. Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J Clin Gastroenterol. 2010; 44:e242–e248.38. Takatsu N, Matsui T, Murakami Y, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2009; 24:1258–1264.
Article39. Ban H, Andoh A, Tanaka A, et al. Analysis of thiopurine S-methyltransferase genotypes in Japanese patients with inflammatory bowel disease. Intern Med. 2008; 47:1645–1648.
Article40. Shah SA, Paradkar M, Desai D, Ashavaid TF. Nucleoside diphosphate-linked moiety X-type motif 15 C415T variant as a predictor for thiopurine-induced toxicity in Indian patients. J Gastroenterol Hepatol. 2017; 32:620–624.
Article41. Schaeffeler E, Fischer C, Brockmeier D, et al. Comprehensive analysis of thiopurine S-methyltransferase phenotype-genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogenetics. 2004; 14:407–417.
Article42. Liu Y, Meng Y, Wang L, Liu Z, Li J, Dong W. Associations between the NUDT15 R139C polymorphism and susceptibility to thiopurine-induced leukopenia in Asians: a meta-analysis. Onco Targets Ther. 2018; 11:8309–8317.43. Relling MV, Schwab M, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019; 105:1095–1105.
Article44. Xin H, Fischer C, Schwab M, Klotz U. Effects of aminosalicylates on thiopurine S-methyltransferase activity: an ex vivo study in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005; 21:1105–1109.
Article45. Szumlanski CL, Weinshilboum RM. Sulphasalazine inhibition of thiopurine methyltransferase: possible mechanism for interaction with 6-mercaptopurine and azathioprine. Br J Clin Pharmacol. 1995; 39:456–459.
Article46. Blaker PA, Arenas-Hernandez M, Smith MA, et al. Mechanism of allopurinol induced TPMT inhibition. Biochem Pharmacol. 2013; 86:539–547.
Article47. Smith MA, Blaker P, Marinaki AM, Anderson SH, Irving PM, Sanderson JD. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohns Colitis. 2012; 6:905–912.
Article48. Sultan KS, Berkowitz JC, Khan S. Combination therapy for inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2017; 8:103–113.
Article49. Atreya I, Diall A, Dvorsky R, et al. Designer thiopurine-analogues for optimised immunosuppression in inflammatory bowel diseases. J Crohns Colitis. 2016; 10:1132–1143.
Article50. Lichtenstein GR, Abreu MT, Cohen R, Tremaine W; American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006; 130:935–939.
Article51. Seinen ML, van Asseldonk DP, Mulder CJ, de Boer NK. Dosing 6-thioguanine in inflammatory bowel disease: expertbased guidelines for daily practice. J Gastrointestin Liver Dis. 2010; 19:291–294.52. Shi HY, Chan FK, Leung WK, et al. Low-dose azathioprine is effective in maintaining remission in steroid-dependent ulcerative colitis: results from a territory-wide Chinese population-based IBD registry. Therap Adv Gastroenterol. 2016; 9:449–456.
Article53. Pal P, Banerjee R. IDDF2018-ABS-0267 Azathioprine for maintenance of steroid-free remission: even a low dose is effective in Indian inflammatory bowel disease (IBD) patients. Gut. 2018; 67(Suppl 2):A87.54. Roblin X, Boschetti G, Williet N, et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: an open-label, prospective and randomised clinical trial. Aliment Pharmacol Ther. 2017; 46:142–149.
Article55. Shih DQ, Nguyen M, Zheng L, et al. Split-dose administration of thiopurine drugs: a novel and effective strategy for managing preferential 6-MMP metabolism. Aliment Pharmacol Ther. 2012; 36:449–458.
Article56. Lee KM, Kim YS, Seo GS, Kim TO, Yang SK; IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of thiopurines in inflammatory bowel disease: a consensus statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015; 13:193–207.
Article57. Pavlidis P, Stamoulos P, Abdulrehman A, et al. Long-term safety and efficacy of low-dose azathioprine and allopurinol cotherapy in inflammatory bowel disease: a large observational study. Inflamm Bowel Dis. 2016; 22:1639–1646.
Article58. Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomized trial. J Crohns Colitis. 2016; 10:1259–1266.
Article59. Hisamatsu T, Kato S, Kunisaki R, et al. Withdrawal of thiopurines in Crohn’s disease treated with scheduled adalimumab maintenance: a prospective randomised clinical trial (DIAMOND2). J Gastroenterol. 2019; 54:860–870.
Article60. Boyapati RK, Torres J, Palmela C, et al. Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn’s disease. Cochrane Database Syst Rev. 2018; 5–CD012540.
Article61. Kang B, Choi SY, Choi YO, et al. Subtherapeutic infliximab trough levels and complete mucosal healing are associated with sustained clinical remission after infliximab cessation in paediatric-onset Crohn’s disease patients treated with combined immunosuppressive therapy. J Crohns Colitis. 2018; 12:644–652.
Article62. Moreau AC, Paul S, Del Tedesco E, et al. Association between 6-thioguanine nucleotides levels and clinical remission in inflammatory disease: a meta-analysis. Inflamm Bowel Dis. 2014; 20:464–471.
Article63. Yarur AJ, Kubiliun MJ, Czul F, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015; 13:1118–1124.
Article64. Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American Gastroenterological Association institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017; 153:835–857.
Article65. Kennedy NA, Asser TL, Mountifield RE, Doogue MP, Andrews JM, Bampton PA. Thiopurine metabolite measurement leads to changes in management of inflammatory bowel disease. Intern Med J. 2013; 43:278–286.
Article66. Beswick L, Friedman AB, Sparrow MP. The role of thiopurine metabolite monitoring in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2014; 8:383–392.
Article67. Bouguen G, Sninsky C, Tang KL, et al. Change in erythrocyte mean corpuscular volume during combination therapy with azathioprine and infliximab is associated with mucosal healing: a post hoc analysis from SONIC. Inflamm Bowel Dis. 2015; 21:606–614.
Article68. Heerasing NM, Ng JF, Dowling D. Does lymphopenia or macrocytosis reflect 6-thioguanine levels in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine? Intern Med J. 2016; 46:465–469.
Article69. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.
Article70. Vasudevan A, Gibson PR, van Langenberg DR. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: what should the clinician expect, what should patients be told? World J Gastroenterol. 2017; 23:6385–6402.
Article71. Matsuoka K. NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease. Intest Res. 2020; 18:275–281.
Article72. Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007; 102:1518–1527.
Article73. Gisbert JP, Luna M, González-Lama Y, et al. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm Bowel Dis. 2007; 13:1106–1114.
Article74. Björnsson ES, Gu J, Kleiner DE, et al. Azathioprine and 6-mercaptopurine-induced liver injury: clinical features and outcomes. J Clin Gastroenterol. 2017; 51:63–69.75. Shamberg L, Vaziri H. Hepatotoxicity of inflammatory bowel disease medications. J Clin Gastroenterol. 2018; 52:674–684.
Article76. Teich N, Mohl W, Bokemeyer B, et al. Azathioprine-induced acute pancreatitis in patients with inflammatory bowel diseases: a prospective study on incidence and severity. J Crohns Colitis. 2016; 10:61–68.
Article77. Bokemeyer B. Asymptomatic elevation of serum lipase and amylase in conjunction with Crohn’s disease and ulcerative colitis. Z Gastroenterol. 2002; 40:5–10.
Article78. Ward MG, Patel KV, Kariyawasam VC, et al. Thioguanine in inflammatory bowel disease: long-term efficacy and safety. United European Gastroenterol J. 2017; 5:563–570.
Article79. Actis GC, Pellicano R, Rosina F. 6-Mercaptopurine for azathioprine intolerant inflammatory bowel disease: literature search and reappraisal of own data. Inflamm Allergy Drug Targets. 2015; 14:133–137.80. Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018; 155:337–346.
Article81. Toruner M, Loftus EV Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008; 134:929–936.
Article82. Rahier JF, Ben-Horin S, Chowers Y, et al. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009; 3:47–91.
Article83. Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol. 2015; 13:847–858.
Article84. Kobayashi T, Uda A, Udagawa E, Hibi T. Lack of increased risk of lymphoma by thiopurines or biologics in Japanese patients with inflammatory bowel disease: a large-scale administrative database analysis. J Crohns Colitis. 2020; 14:617–623.85. Beaugerie L. Lymphoma: the bête noire of the long-term use of thiopurines in adult and elderly patients with inflammatory bowel disease. Gastroenterology. 2013; 145:927–930.
Article86. Lopez A, Mounier M, Bouvier AM, et al. Increased risk of acute myeloid leukemias and myelodysplastic syndromes in patients who received thiopurine treatment for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014; 12:1324–1329.
Article87. Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2014; 109:163–169.
Article88. Hutfless S, Fireman B, Kane S, Herrinton LJ. Screening differences and risk of cervical cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2008; 28:598–605.
Article89. Bourrier A, Carrat F, Colombel JF, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther. 2016; 43:252–261.90. Akbari M, Shah S, Velayos FS, Mahadevan U, Cheifetz AS. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013; 19:15–22.
Article91. Hutson JR, Matlow JN, Moretti ME, Koren G. The fetal safety of thiopurines for the treatment of inflammatory bowel disease in pregnancy. J Obstet Gynaecol. 2013; 33:1–8.
Article92. Bröms G, Granath F, Linder M, Stephansson O, Elmberg M, Kieler H. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis. 2014; 20:1091–1098.93. Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019; 156:1508–1524.
Article94. Juneja M, Baidoo L, Schwartz MB, et al. Geriatric inflammatory bowel disease: phenotypic presentation, treatment patterns, nutritional status, outcomes, and comorbidity. Dig Dis Sci. 2012; 57:2408–2415.95. Levine A, Koletzko S, Turner D, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014; 58:795–806.
Article96. Riello L, Talbotec C, Garnier-Lengliné H, et al. Tolerance and efficacy of azathioprine in pediatric Crohn’s disease. Inflamm Bowel Dis. 2011; 17:2138–2143.
Article97. Turner D, Levine A, Escher JC, et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidencebased consensus guidelines. J Pediatr Gastroenterol Nutr. 2012; 55:340–361.98. Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014; 8:1179–1207.99. Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J Crohns Colitis. 2020; 14:1334–1336.100. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395:1033–1034.
Article101. Khan N, Patel D, Xie D, Lewis J, Trivedi C, Yang YX. Impact of anti-tumor necrosis factor and thiopurine medications on the development of COVID-19 in patients with inflammatory bowel disease: a nationwide veterans administration cohort study. Gastroenterology. 2020; 159:1545–1546.
Article102. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020; 159:481–491.
Article103. Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. 2020; 159:350–357.
Article104. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017; 112:241–258.105. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014; 58:309–318.
Article106. Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel JF, Satsangi J. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology. 2015; 149:1716–1730.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monitoring and Safety of Azathioprine Therapy in Inflammatory Bowel Disease
- Use of Thiopurines in Inflammatory Bowel Disease: A Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID)
- Inflammatory Bowel Disease and Lymphoproliferative Disorders
- Update on the epidemiology of inflammatory bowel disease in Asia: where are we now?
- Treatment of inflammatory bowel diseases: focusing on 5-aminosalicylates and immunomodulators