Healthc Inform Res.
2021 Oct;27(4):341-349. 10.4258/hir.2021.27.4.341.
Research Development Using REDCap Software
- Affiliations
-
- 1Tropical Medicine Nucleus, Universidade de Brasília, Brasília, Brazil
- 2Ministry of Health, Brasília, Brazil
- KMID: 2522216
- DOI: http://doi.org/10.4258/hir.2021.27.4.341
Abstract
Objectives
High-quality clinical research is dependent on adequate design, methodology, and data collection. The utilization of electronic data capture (EDC) systems is recommended to optimize research data through proper management. This paper’s objective is to present the procedures of REDCap (Research Electronic Data Capture), which supports research development, and to promote the utilization of this software among the scientific community.
Methods
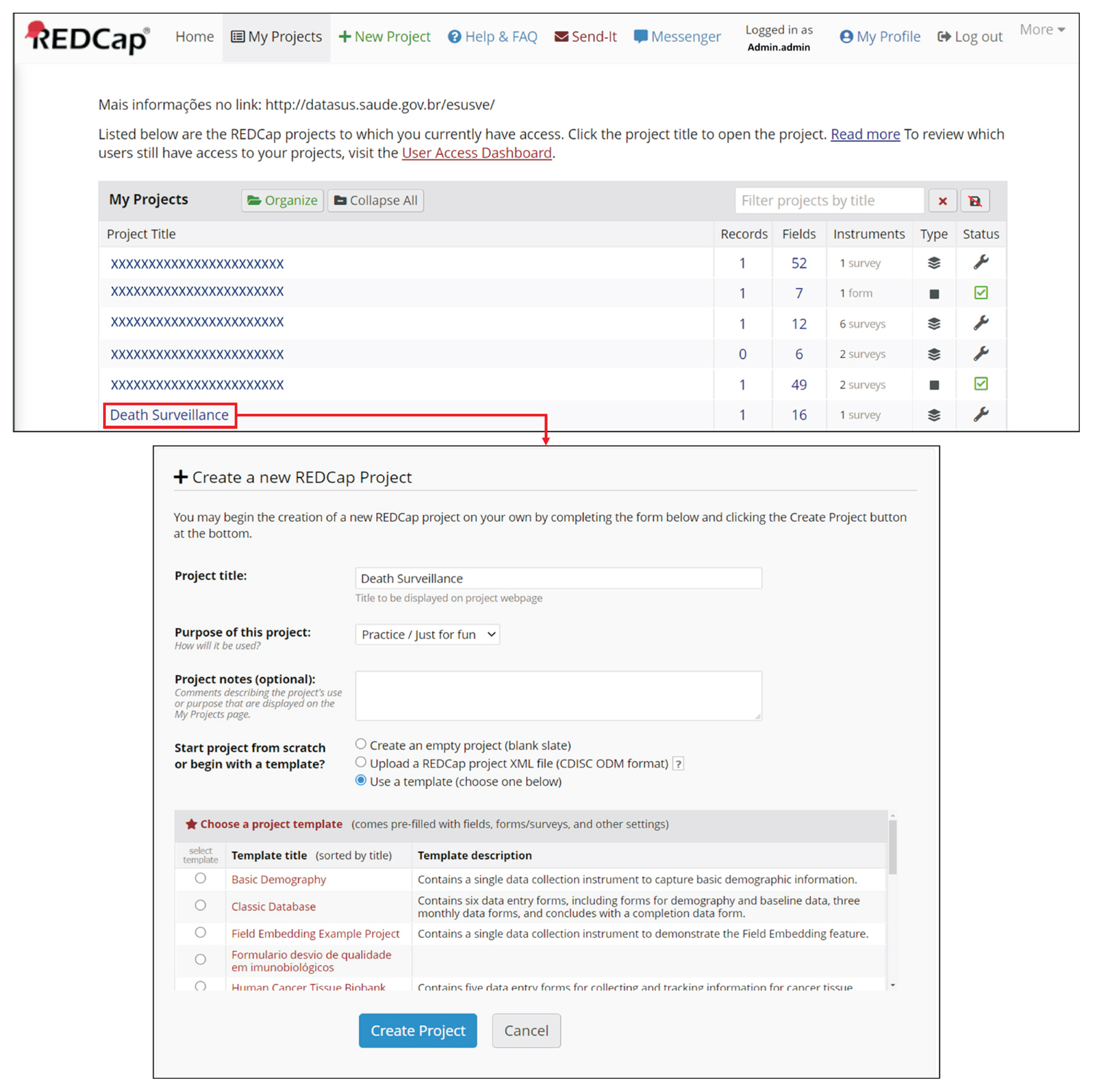
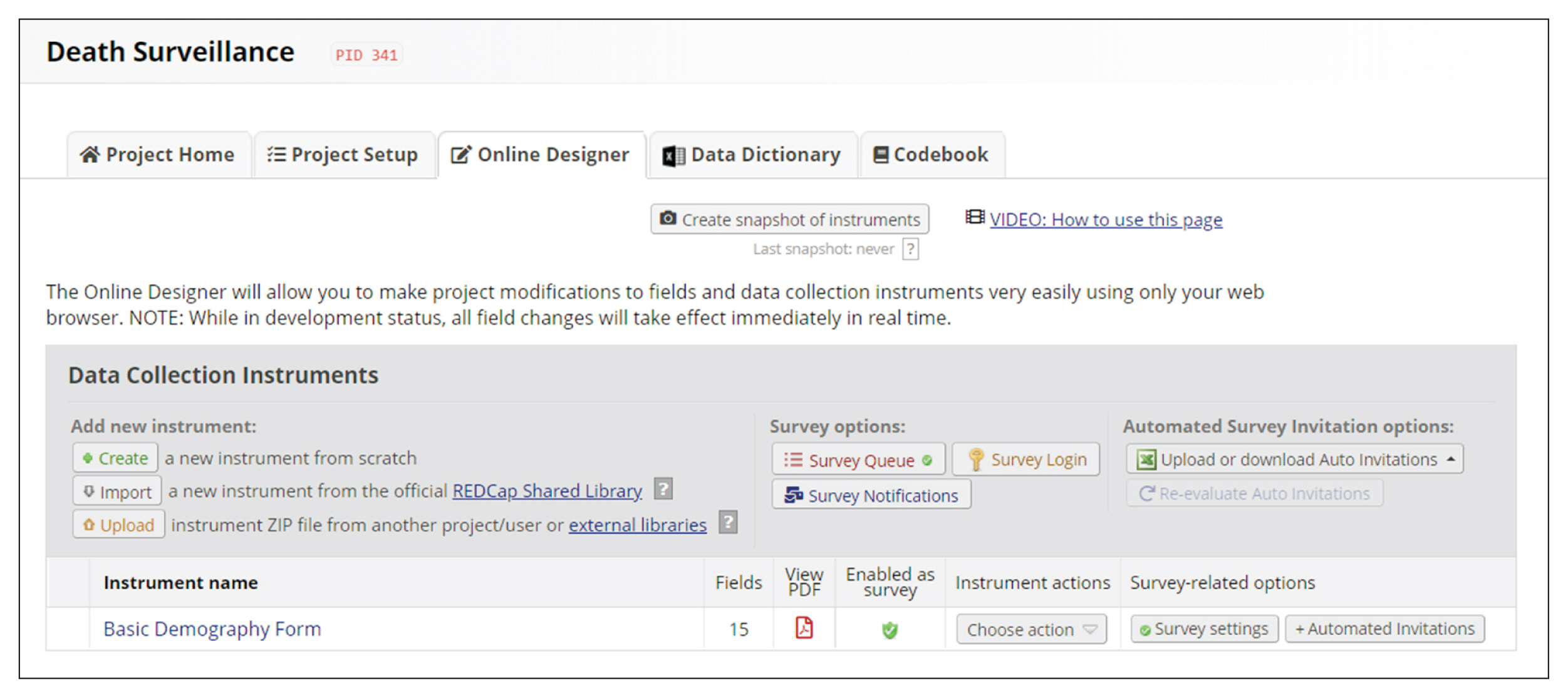
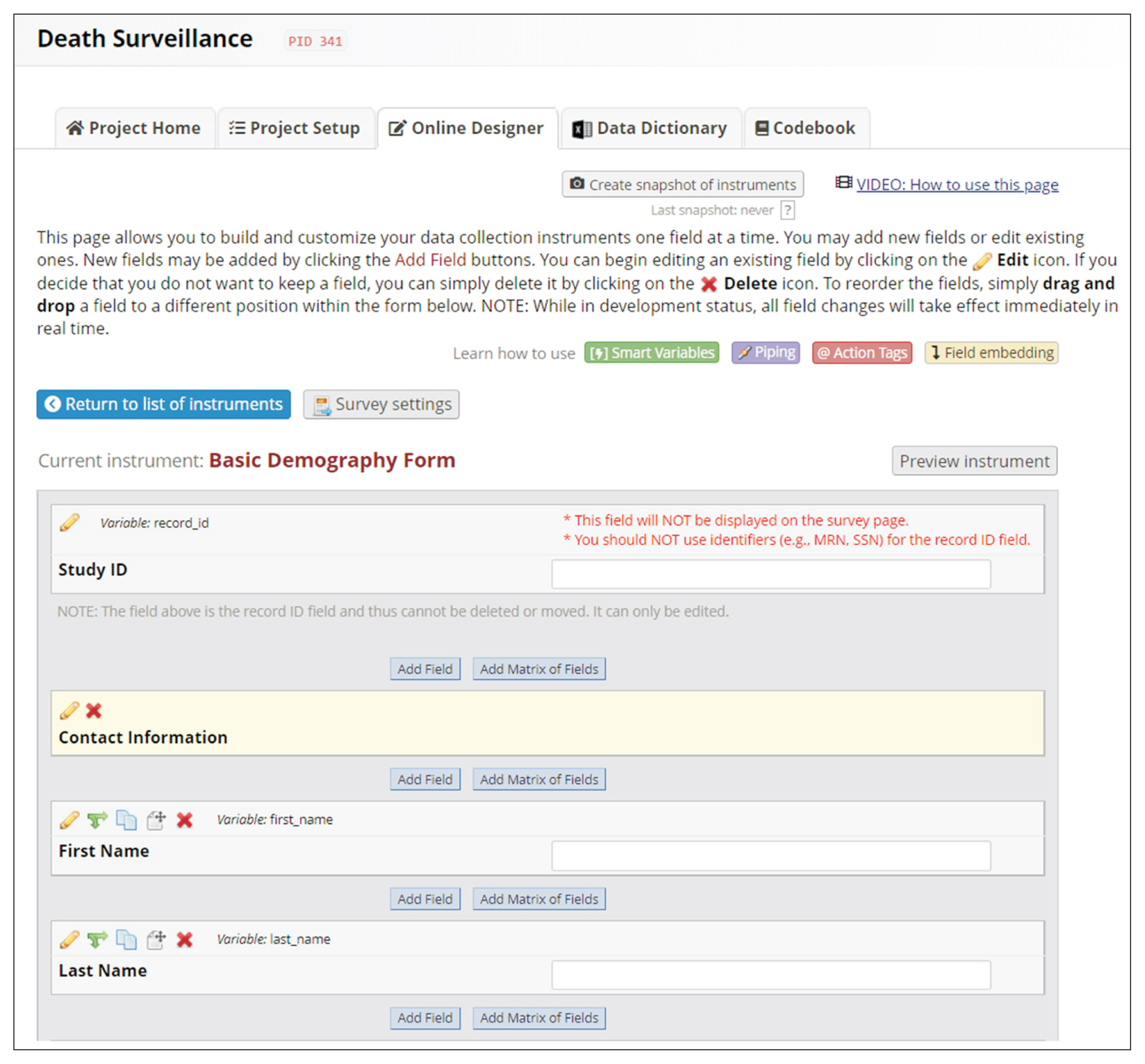
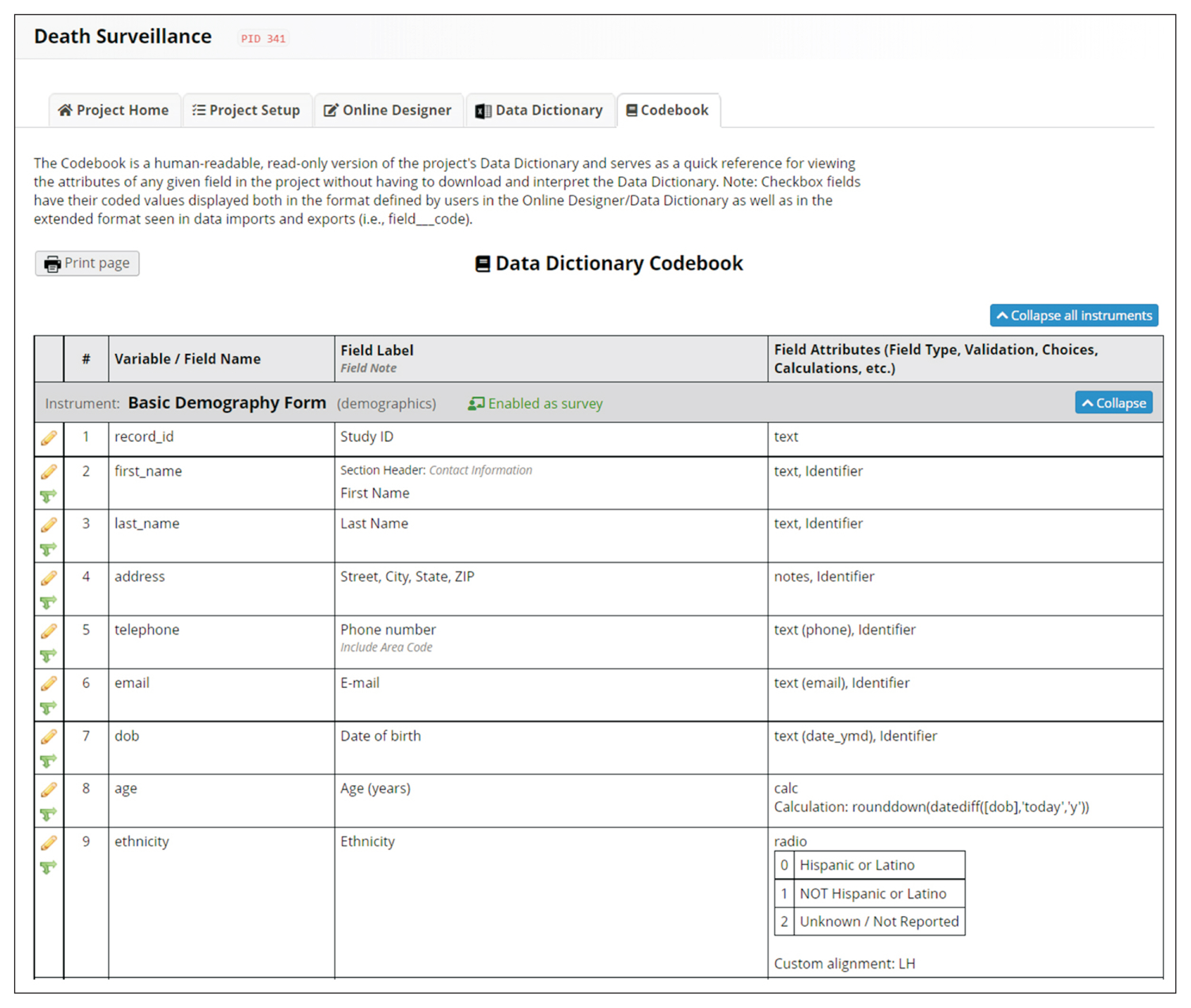
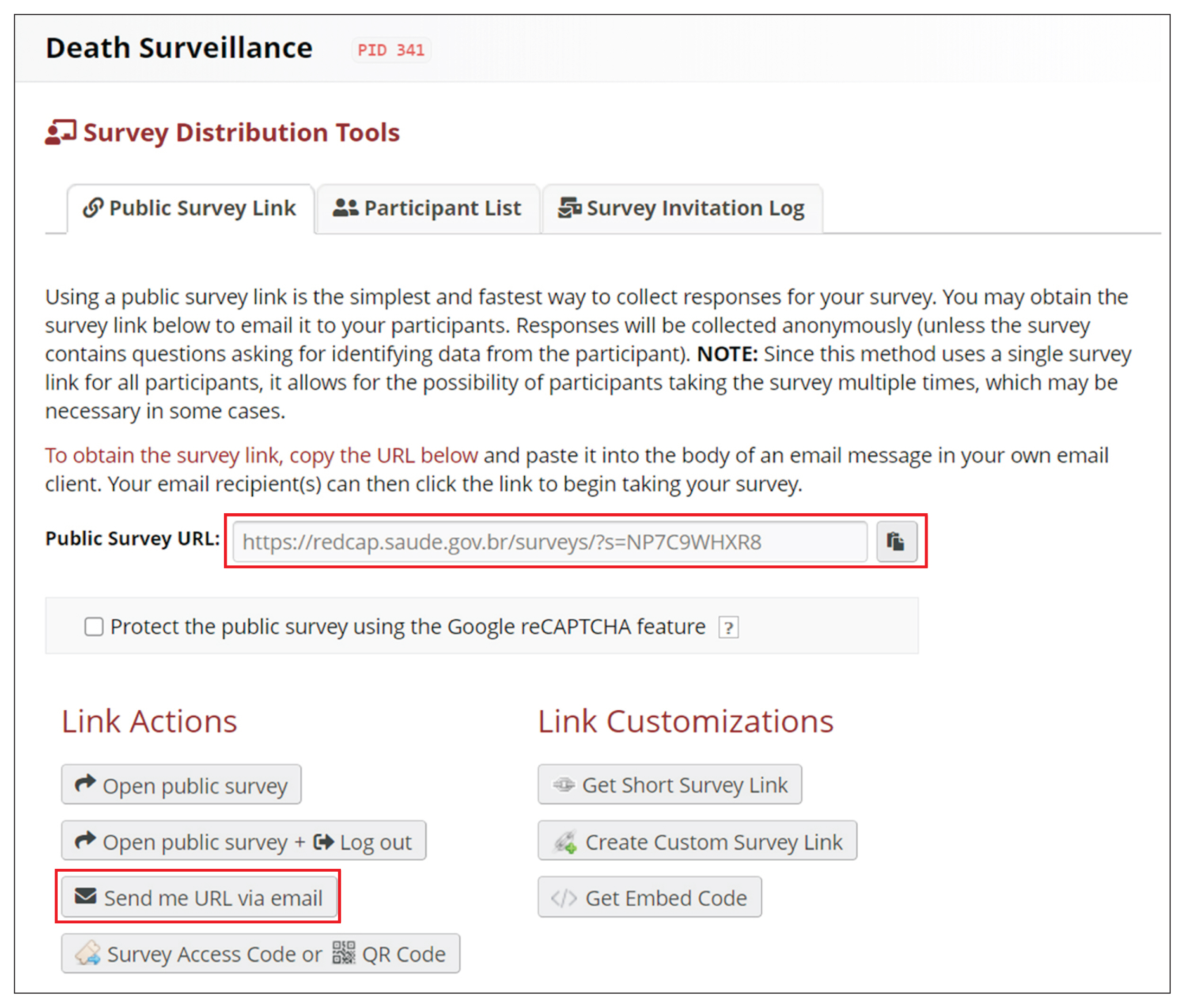
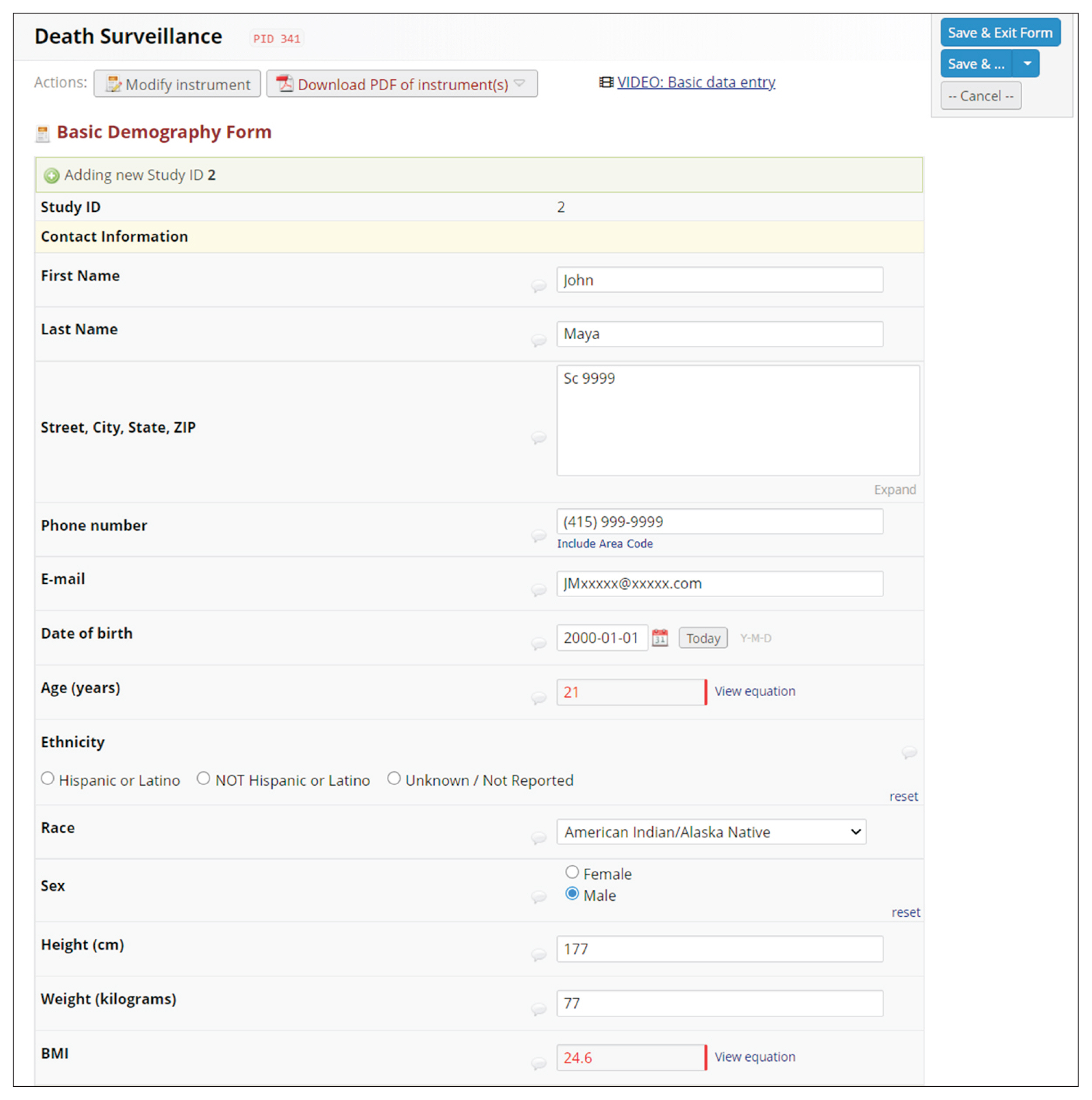
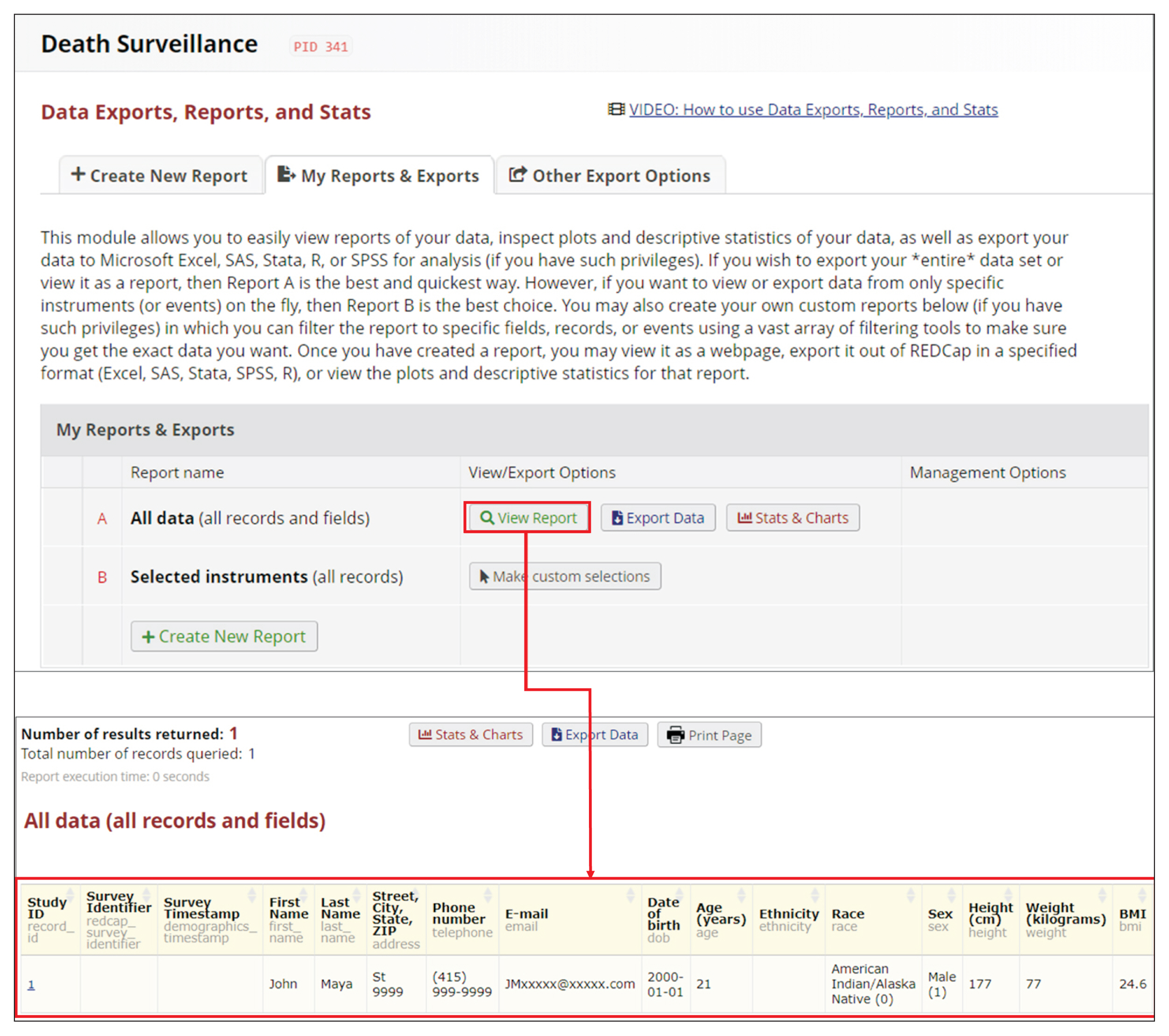
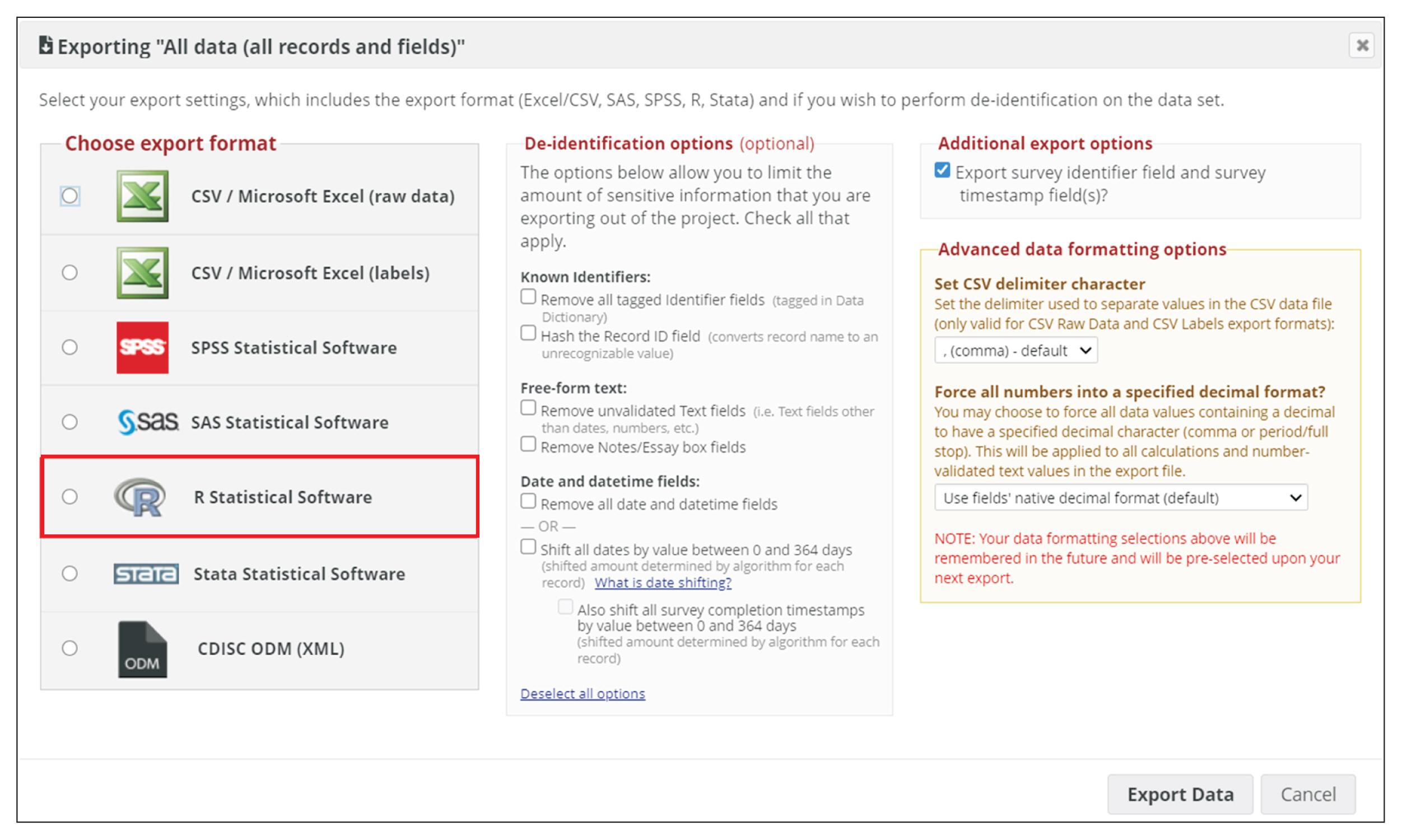
REDCap’s web application version 10.4.1 released on 2021 (Vanderbilt University) is an EDC system suitable for clinical research development. This paper describes how to join the REDCap consortium and presents how to develop survey instruments and use them to collect and analyze data.
Results
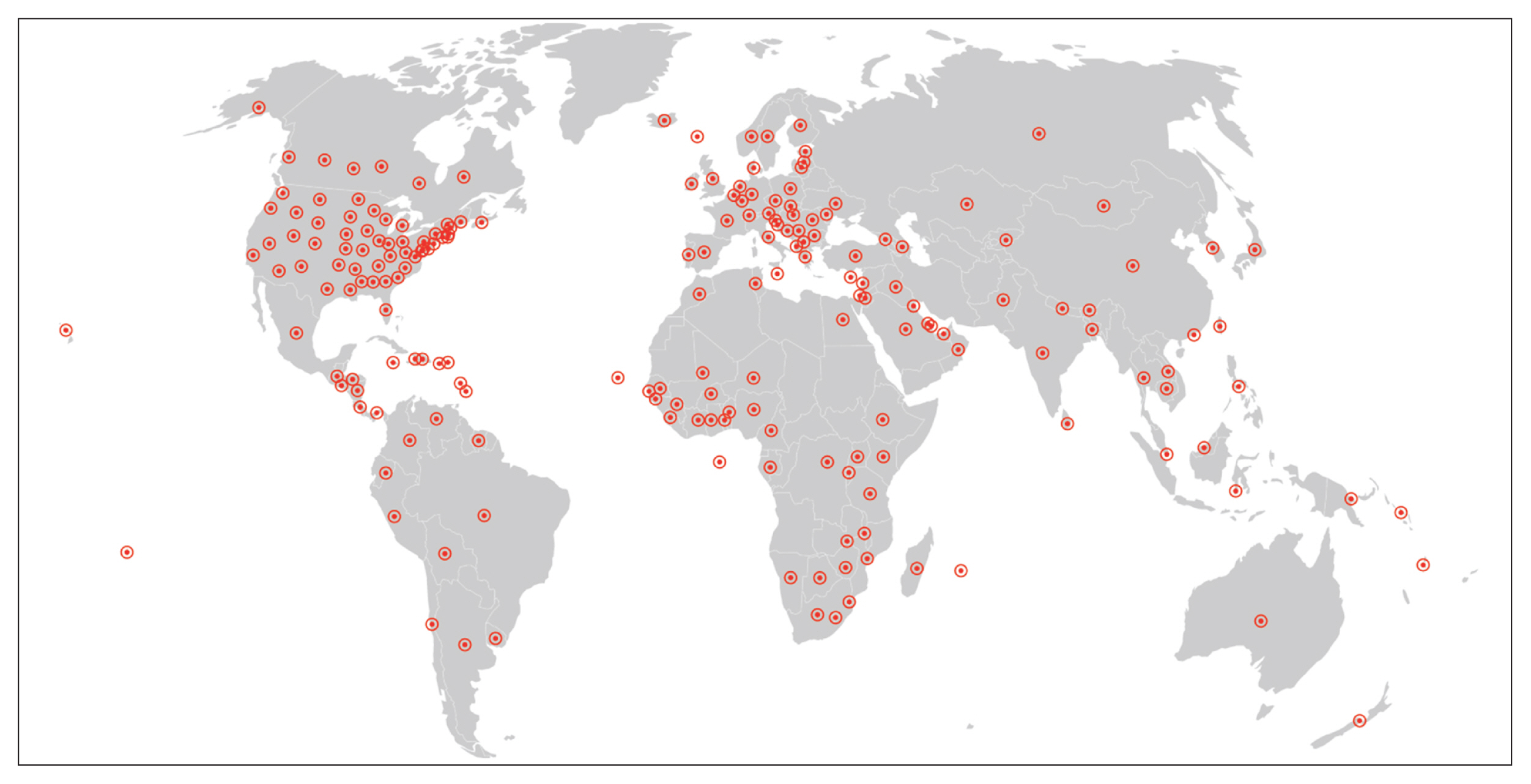
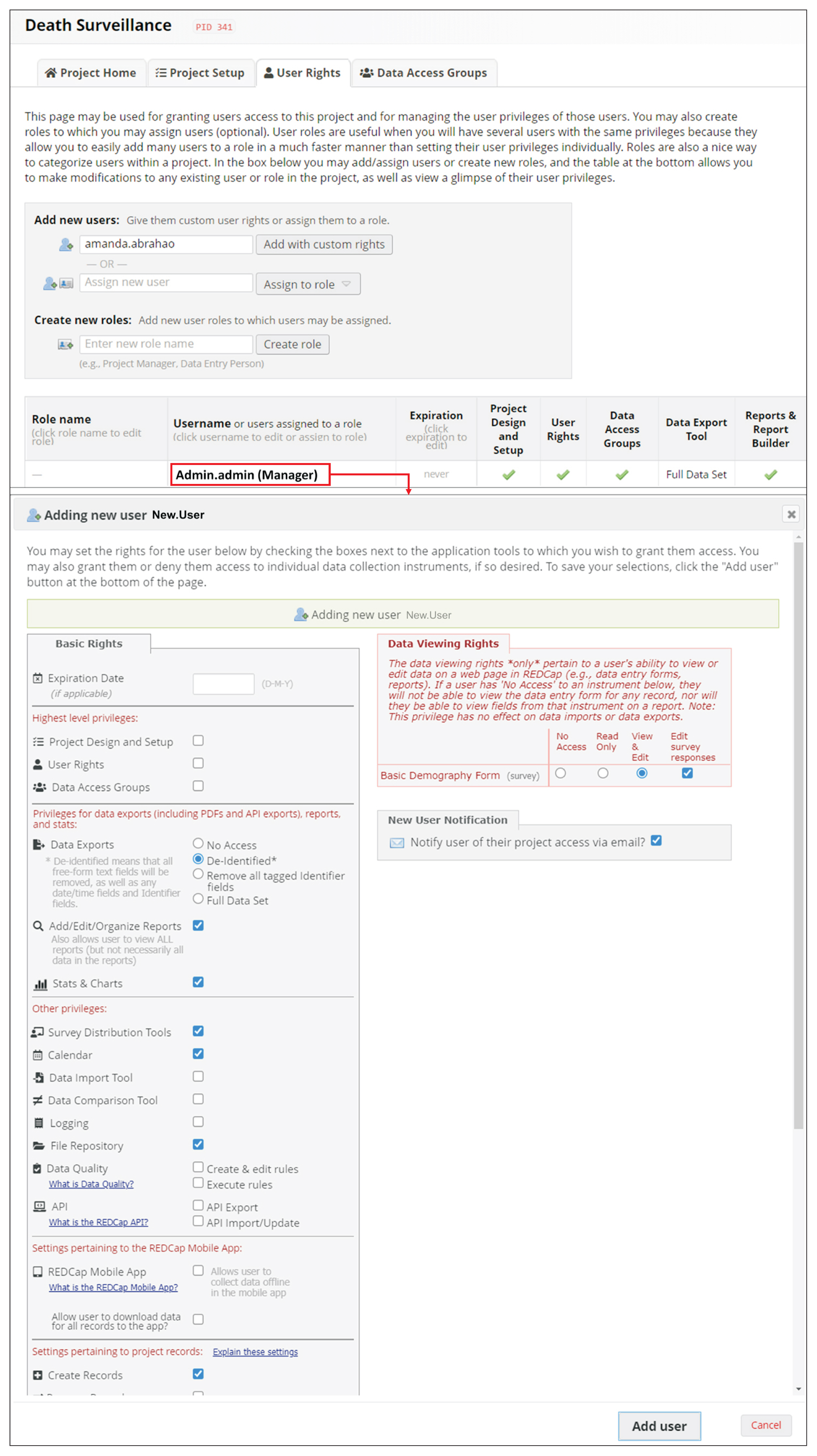
Since REDCap is a web application that stimulates knowledge-sharing among the scientific community, its development is not finished and it is constantly receiving updates to improve the system. REDCap’s tools provide access control, audit trails, and data security to the research team.
Conclusions
REDCap is a web application that can facilitate clinical research development, mainly in health fields, and reduce the costs of conducting research. Its tools allow researchers to make the best use of EDC components, such as data storage.
Figure
Reference
-
References
1. Haug A, Zachariassen F, Van Liempd D. The costs of poor data quality. J Ind Eng Manag. 2011; 4(2):168–93.
Article2. World Health Organization. Report of the technical discussions at the twenty-first World Health Assembly on “national and global surveillance of communicable diseases”. Geneva, Switzerland: World Health Organization;1968.3. Choi BC. The past, present, and future of public health surveillance. Scientifica (Cairo). 2012; 2012:875253.
Article4. Backman C, Vanderloo S, Momtahan K, d’Entremont B, Freeman L, Kachuik L, et al. Implementation of an electronic data collection tool to monitor nursing-sensitive indicators in a large academic health sciences centre. Nurs Leadersh (Tor Ont). 2015; 28(3):77–91.5. Haux R. Health information systems: past, present, future. Int J Med Inform. 2006; 75(3–4):268–81.6. Walther B, Hossin S, Townend J, Abernethy N, Parker D, Jeffries D. Comparison of electronic data capture (EDC) with the standard data capture method for clinical trial data. PLoS One. 2011; 6(9):e25348.
Article7. Patridge EF, Bardyn TP. Research electronic data capture (REDCap). J Med Libr Assoc. 2018; 106(1):142–4.
Article8. Oliveira SB, Ganem F, Araujo WN, Casabona J, Sanchez MN, Croda J. Imputation method to reduce undetected severe acute respiratory infection cases during the coronavirus disease outbreak in Brazil. Rev Soc Bras Med Trop. 2020; 53:e20200528.
Article9. Rufalco-Moutinho P, de Noronha LA, Quintao TD, Nobre TF, Cardoso AP, Ciliao-Alves DC, et al. Evidence of co-circulation of multiples arbovirus transmitted by Aedes Sp. based on laboratorial syndromic surveillance at health unit in slum area of federal district, Brazil [Internet]. Durham (NC): Research Square;2021. [cited at 2021 Oct 6]. Available from: https://www.research-square.com/article/rs-588530/v1 .10. Duarte MM, Haslett MI, Freitas LJ, Gomes NT, Silva DC, Percio J, et al. Description of COVID-19 hospitalized health worker cases in the first nine weeks of the pandemic, Brazil, 2020. Epidemiol Serv Saude. 2020; 29(5):e2020277.11. Nishio EA, Cardoso ML, Salvador ME, D’Innocenzo M. Evaluation of Nursing Service Management Model applied in hospitals managed by social health organization. Rev Bras Enferm. 2021; 74(suppl 5):e20200876.
Article12. Leiria TL, Santos CB, Sant’anna RT, Trombetta JS, Osterkamp G, Kruse ML, et al. Analysis of conduction intervals in normal electrophysiological studies: establishment of reference values the Brazilian population. Int J Cardiovasc Sci. 2000; 33:488–94.13. Vaz J, Abelin AP, Schmidt MM, Oliveira PP, Gottschall CA, Rodrigues CG, et al. Creation and implementation of a prospective and multicentric database of patients with acute myocardial infarction: RIAM. Arq Bras Cardiol. 2020; 114(3):446–55.14. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019; 95:103208.
Article15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42(2):377–81.16. R Core Team. R: a language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing;c2021. [cited 2021 Oct 5]. Available from: https://www.R-project.org/ .17. de Souza WM, Buss LF, Candido DD, Carrera JP, Li S, Zarebski AE, et al. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat Hum Behav. 2020; 4(8):856–65.
Article18. Gadsden T, Bateman-Steel CR, Chaverot S, Ressler KA, Chee K, Redwood L, et al. Using a computerised database (REDCap) to monitor influenza vaccination coverage of healthcare workers and staff in South Eastern Sydney Local Health District. Aust Health Rev. 2021; 45(1):97–103.
Article19. Maguire BJ, Guerin PJ. A living systematic review protocol for COVID-19 clinical trial registrations. Wellcome Open Res. 2020; 5:60.
Article20. Marcolino MS, Ziegelmann PK, Souza-Silva MV, Nascimento IJ, Oliveira LM, Monteiro LS, et al. Clinical characteristics and outcomes of patients hospitalized with COVID-19 in Brazil: results from the Brazilian COVID-19 registry. Int J Infect Dis. 2021; 107:300–10.21. Koetter P, Pelton M, Gonzalo J, Du P, Exten C, Bogale K, et al. Implementation and process of a COVID-19 contact tracing initiative: leveraging health professional students to extend the workforce during a pandemic. Am J Infect Control. 2020; 48(12):1451–6.
Article22. Gomides A, Ferreira G, Kakehasi A, Lacerda M, Marques C, Mota L, et al. Impact of chronic use of antimalarials on SARS-CoV-2 infection in patients with immune-mediated rheumatic diseases: protocol for a multicentric observational cohort study. JMIR Res Protoc. 2020; 9(10):e23532.
Article23. Crane S, Comer RS, Arenson AD, Draucker C. Using REDCap to facilitate web-based therapeutic intervention research. Nurs Res. 2019; 68(6):483–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Construction of a Retrospective Cohort to Observe 10-Year Urologic Cancer Treatment Trends at the Biggest Medical Center of South Korea
- Development of Software Solutions for Stroke: A Personal Experience
- Design and Construction of a NLP Based Knowledge Extraction Methodology in the Medical Domain Applied to Clinical Information
- Brain MRI-Based Artificial Intelligence Software in Patients with Neurodegenerative Diseases: Current Status
- Development of PC-based Software to Analyze Dynamic Cerebral Perfusion CT Quantitatively and to Reformat Perfusion Maps