Endocrinol Metab.
2021 Oct;36(5):1078-1085. 10.3803/EnM.2021.1151.
Clinicopathological Characteristics and Disease-Free Survival in Patients with Hürthle Cell Carcinoma: A Multicenter Cohort Study in South Korea
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 4Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea
- 5Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University College of Medicine, Daejeon, Korea
- KMID: 2521955
- DOI: http://doi.org/10.3803/EnM.2021.1151
Abstract
- Background
Hürthle cell carcinoma (HCC), a type of thyroid carcinoma, is rare in South Korea, and few studies have investigated its prognosis.
Methods
This long-term multicenter retrospective cohort study evaluated the clinicopathological features and clinical outcomes in patients with HCC who underwent thyroid surgery between 1996 and 2009.
Results
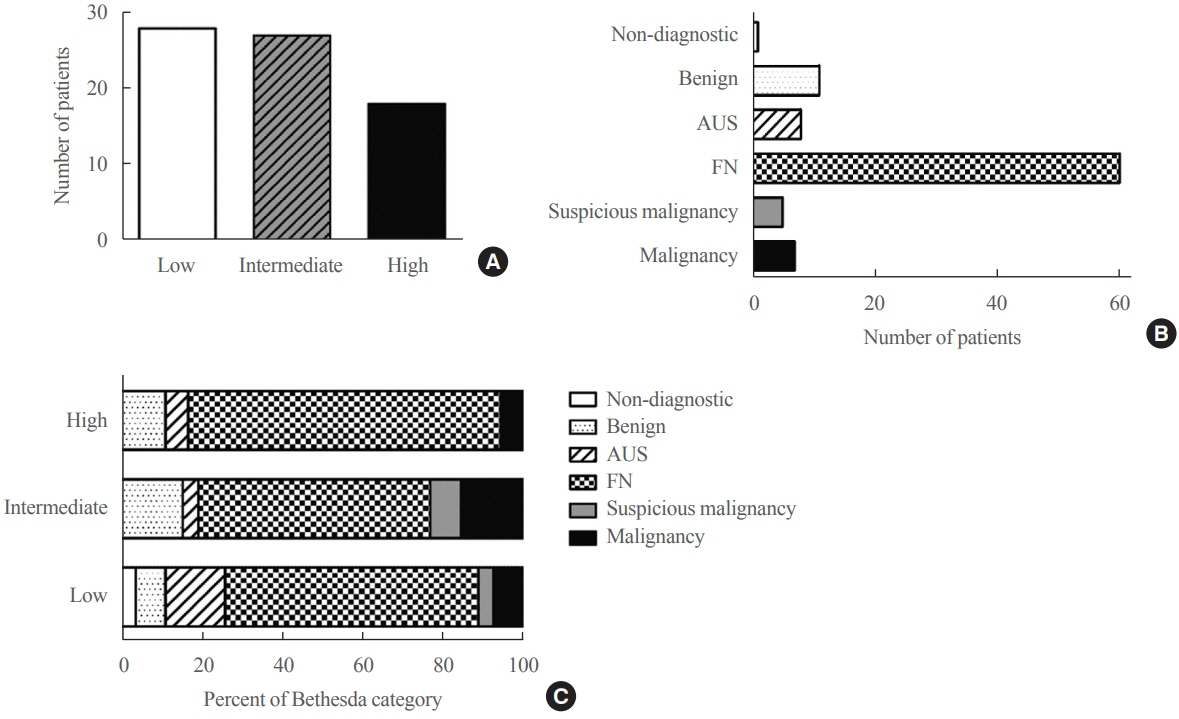
The mean age of the 97 patients included in the study was 50.3 years, and 26.8% were male. The mean size of the primary tumor was 3.2±1.8 cm, and three (3.1%) patients had distant metastasis at initial diagnosis. Ultrasonographic findings were available for 73 patients; the number of nodules with low-, intermediate-, and high suspicion was 28 (38.4%), 27 (37.0%), and 18 (24.7%), respectively, based on the Korean-Thyroid Imaging Reporting and Data System. Preoperatively, follicular neoplasm (FN) or suspicion for FN accounted for 65.2% of the cases according to the Bethesda category, and 13% had malignancy or suspicious for malignancy. During a median follow-up of 8.5 years, eight (8.2%) patients had persistent/recurrent disease, and none died of HCC. Older age, gross extrathyroidal extension (ETE), and widely invasive types of tumors were significantly associated with distant metastasis (all P<0.01). Gross ETE (hazard ratio [HR], 27.7; 95% confidence interval [CI], 2.2 to 346.4; P=0.01) and widely invasive classification (HR, 6.5; 95% CI, 1.1 to 39.4; P=0.04) were independent risk factors for poor disease-free survival (DFS).
Conclusion
The long-term prognosis of HCC is relatively favorable in South Korea from this study, although this is not a nation-wide data, and gross ETE and widely invasive cancer are significant prognostic factors for DFS. The diagnosis of HCC by ultrasonography and cytopathology remains challenging.
Figure
Reference
-
1. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer. 1998; 83:2638–48.
Article2. Hundahl SA, Cady B, Cunningham MP, Mazzaferri E, McKee RF, Rosai J, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German Thyroid Cancer Study Group: an American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer. 2000; 89:202–17.
Article3. Mete O, Asa SL. Oncocytes, oxyphils, Hurthle, and Askanazy cells: morphological and molecular features of oncocytic thyroid nodules. Endocr Pathol. 2010; 21:16–24.
Article4. Montone KT, Baloch ZW, LiVolsi VA. The thyroid Hurthle (oncocytic) cell and its associated pathologic conditions: a surgical pathology and cytopathology review. Arch Pathol Lab Med. 2008; 132:1241–50.5. Kure S, Ohashi R. Thyroid Hurthle cell carcinoma: clinical, pathological, and molecular features. Cancers (Basel). 2020; 13:26.
Article6. Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989; 63:908–11.
Article7. Ganly I, Ricarte Filho J, Eng S, Ghossein R, Morris LG, Liang Y, et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J Clin Endocrinol Metab. 2013; 98:E962–72.
Article8. LIoyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 10th ed. Lyon: International Agency for Research on Cancer;2017.9. Goffredo P, Roman SA, Sosa JA. Hurthle cell carcinoma: a population-level analysis of 3311 patients. Cancer. 2013; 119:504–11.10. Phitayakorn R, McHenry CR. Follicular and Hurthle cell carcinoma of the thyroid gland. Surg Oncol Clin N Am. 2006; 15:603–23.11. Lopez-Penabad L, Chiu AC, Hoff AO, Schultz P, Gaztambide S, Ordonez NG, et al. Prognostic factors in patients with Hurthle cell neoplasms of the thyroid. Cancer. 2003; 97:1186–94.
Article12. Nagar S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, Grogan RH. Hurthle cell carcinoma: an update on survival over the last 35 years. Surgery. 2013; 154:1263–71.
Article13. Khafif A, Khafif RA, Attie JN. Hurthle cell carcinoma: a malignancy of low-grade potential. Head Neck. 1999; 21:506–11.
Article14. Bhattacharyya N. Survival and prognosis in Hurthle cell carcinoma of the thyroid gland. Arch Otolaryngol Head Neck Surg. 2003; 129:207–10.
Article15. Kim WG, Yim JH, Kim EY, Kim TY, Gong G, Yoon JH, et al. Preoperative cytological diagnosis of follicular thyroid carcinoma and Huthle cell carcinoma. J Korean Thyroid Assoc. 2010; 3:149–54.16. Kim WG, Kim TY, Kim TH, Jang HW, Jo YS, Park YJ, et al. Follicular and Hurthle cell carcinoma of the thyroid in iodine-sufficient area: retrospective analysis of Korean multicenter data. Korean J Intern Med. 2014; 29:325–33.
Article17. Kim TH, Lim JA, Ahn HY, Lee EK, Min HS, Kim KW, et al. Tumor size and age predict the risk of malignancy in Hurthle cell neoplasm of the thyroid and can therefore guide the extent of initial thyroid surgery. Thyroid. 2010; 20:1229–34.
Article18. Santana NO, Freitas RM, Marcos VN, Chammas MC, Camargo RY, Schmerling CK, et al. Diagnostic performance of thyroid ultrasound in Hurthle cell carcinomas. Arch Endocrinol Metab. 2019; 63:300–5.19. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–95.
Article20. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017; 27:1341–6.
Article21. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article22. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–214.
Article23. Jin M, Kim ES, Kim BH, Kim HK, Yi HS, Jeon MJ, et al. Clinical implication of World Health Organization classification in patients with follicular thyroid carcinoma in South Korea: a multicenter cohort study. Endocrinol Metab (Seoul). 2020; 35:618–27.
Article24. Mills SC, Haq M, Smellie WJ, Harmer C. Hurthle cell carcinoma of the thyroid: retrospective review of 62 patients treated at the Royal Marsden Hospital between 1946 and 2003. Eur J Surg Oncol. 2009; 35:230–4.25. Oluic B, Paunovic I, Loncar Z, Djukic V, Diklic A, Jovanovic M, et al. Survival and prognostic factors for survival, cancer specific survival and disease free interval in 239 patients with Hurthle cell carcinoma: a single center experience. BMC Cancer. 2017; 17:371.
Article26. Chindris AM, Casler JD, Bernet VJ, Rivera M, Thomas C, Kachergus JM, et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab. 2015; 100:55–62.
Article27. Baloch ZW, LiVolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:425–37.
Article28. Sorrenti S, Trimboli P, Catania A, Ulisse S, De Antoni E, D’Armiento M. Comparison of malignancy rate in thyroid nodules with cytology of indeterminate follicular or indeterminate Hurthle cell neoplasm. Thyroid. 2009; 19:355–60.
Article29. Pu RT, Yang J, Wasserman PG, Bhuiya T, Griffith KA, Michael CW. Does Hurthle cell lesion/neoplasm predict malignancy more than follicular lesion/neoplasm on thyroid fine-needle aspiration? Diagn Cytopathol. 2006; 34:330–4.
Article30. Thompson NW, Dunn EL, Batsakis JG, Nishiyama RH. Hurthle cell lesions of the thyroid gland. Surg Gynecol Obstet. 1974; 139:555–60.31. Yutan E, Clark OH. Hurthle cell carcinoma. Curr Treat Options Oncol. 2001; 2:331–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Analysis About Clinical Manifestation and Prognostic Factors of Thyroid Follicular and Hurthle Cell Carcinoma
- Hurthle Cell Carcinoma of the Thyroid: A Case Report of 12 Cases
- Hurthle Cell Carcinoma of the Thyroid Gland: Clinicopathologic Features and Treatment Outcome Compared with Pure Follicular Thyroid Carcinoma
- Relationship between Clinicopathological Characteristics and Telomerase Activity of Renal Cell Carcinoma
- Cytopathology of Hurthle Cell Adenoma: A Cese Report by Fine Needle Aspiration