Ann Pediatr Endocrinol Metab.
2021 Jun;26(2):105-111. 10.6065/apem.2040150.075.
Efficacy and safety of intravenous pamidronate infusion for treating osteoporosis in children and adolescents
- Affiliations
-
- 1Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2517588
- DOI: http://doi.org/10.6065/apem.2040150.075
Abstract
- Purpose
Osteoporosis is a skeletal disorder characterized by reduced bone mass that results in increased risk of fractures. Pediatric osteoporosis can be caused by monogenic diseases, chronic diseases, and/or their treatment. This study was performed to investigate the effect of pamidronate infusion on osteoporosis in children and adolescents.
Methods
This study included 13 unrelated pediatric patients (10 males and 3 females) whose bone mineral density (BMD) z-score was <-2.0. Pamidronate was administered intravenously at a dosage of 1 mg/kg for 3 consecutive days every 4 months. Clinical and biochemical findings were reviewed retrospectively. The BMD values of the lumbar spine and femoral neck were assessed by dual energy x-ray absorptiometry at baseline and annually.
Results
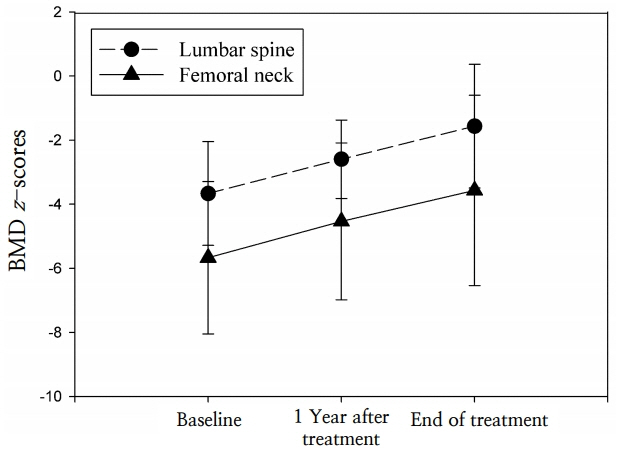
The underlying diseases were immobilization (62%), inflammatory bowel disease (23%), protein-losing enteropathy (8%), and idiopathic juvenile osteoporosis (8%). The mean age at the start of treatment was 12.7±4.3 years. Duration of treatment ranged from 12–50 months. The baseline height-standard deviation score (SDS) and weight-SDS were -2.01±2.08 and -2.60±1.62, respectively. The lumbar spine BMD z-scores improved significantly after 1 year of pamidronate treatment, but the femoral neck BMD z-scores did not. However, both z-scores had significantly increased by the end of treatment.
Conclusion
This study demonstrated that pamidronate treatment increased BMD in pediatric patients with osteoporosis with no significant adverse events. Further studies are required to better define the long-term efficacy and safety of pamidronate therapy in a large number of pediatric patients.
Figure
Cited by 1 articles
-
Effectiveness and safety of pamidronate treatment in nonambulatory children with low bone mineral density
Myeongseob Lee, Ahreum Kwon, Kyungchul Song, Hae In Lee, Han Saem Choi, Junghwan Suh, Hyun Wook Chae, Ho-Seong Kim
Ann Pediatr Endocrinol Metab. 2024;29(1):46-53. doi: 10.6065/apem.2346028.014.
Reference
-
References
1. Marrani E, Giani T, Simonini G, Cimaz R. Pediatric osteoporosis: diagnosis and treatment considerations. Drugs. 2017; 77:679–95.
Article2. Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, et al. Executive summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J Clin Densitom. 2019; 22:453–71.
Article3. Arundel P, Bishop N. Primary osteoporosis. Endocr Dev. 2015; 28:162–75.
Article4. Ward LM, Konji VN, Ma J. The management of osteoporosis in children. Osteoporos Int. 2016; 27:2147–79.
Article5. Bianchi ML. Osteoporosis in children and adolescents. Bone. 2007; 41:486–95.
Article6. Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007; 119 Suppl 2:S150–62.
Article7. Eghbali-Fatourechi G. Bisphosphonate therapy in pediatric patients. J Diabetes Metab Disord. 2014; 13:109.
Article8. Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998; 339:947–52.
Article9. Henderson RC, Lark RK, Kecskemethy HH, Miller F, Harcke HT, Bachrach SJ. Bisphosphonates to treat osteopenia in children with quadriplegic cerebral palsy: a randomized, placebo-controlled clinical trial. J Pediatr. 2002; 141:644–51.
Article10. Acott PD, Wong JA, Lang BA, Crocker JF. Pamidronate treatment of pediatric fracture patients on chronic steroid therapy. Pediatr Nephrol. 2005; 20:368–73.
Article11. Choi JH, Shin YL, Yoo HW. Short-term efficacy of monthly pamidronate infusion in patients with osteogenesis imperfecta. J Korean Med Sci. 2007; 22:209–12.
Article12. Lim SW, Ahn JH, Choi A, Cho WH, Lee JA, Kim DH, et al. Efficacy of pamidronate in pediatric osteosarcoma patients with low bone mineral density. Ann Pediatr Endocrinol Metab. 2016; 21:21–5.
Article13. Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Bone Miner Res. 2009; 24:1282–9.
Article14. Ooi HL, Briody J, McQuade M, Munns CF. Zoledronic acid improves bone mineral density in pediatric spinal cord injury. J Bone Miner Res. 2012; 27:1536–40.
Article15. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.
Article16. Lim JS, Hwang JS, Lee JA, Kim DH, Park KD, Cheon GJ, et al. Bone mineral density according to age, bone age, and pubertal stages in korean children and adolescents. J Clin Densitom. 2010; 13:68–76.
Article17. Kang MJ, Hong HS, Chung SJ, Lee YA, Shin CH, Yang SW. Body composition and bone density reference data for Korean children, adolescents, and young adults according to age and sex: results of the 2009-2010 Korean National Health and Nutrition Examination Survey (KNHANES). J Bone Miner Metab. 2016; 34:429–39.
Article18. Fan B, Lu Y, Genant H, Fuerst T, Shepherd J. Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporos Int. 2010; 21:1227–36.
Article19. Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev. 2014; 10:CD005088.
Article20. Saraff V, Hogler W. Endocrinology and adolescence: osteoporosis in children: diagnosis and management. Eur J Endocrinol. 2015; 173:R185–97.
Article21. Jung KJ, Kwon SS, Chung CY, Lee KM, Sung KH, Cho BC, et al. Association of gross motor function classification system level and school attendance with bone mineral density in patients with cerebral palsy. J Clin Densitom. 2018; 21:501–6.
Article22. Grissom LE, Kecskemethy HH, Bachrach SJ, McKay C, Harcke HT. Bone densitometry in pediatric patients treated with pamidronate. Pediatr Radiol. 2005; 35:511–7.
Article23. Baroncelli GI, Vierucci F, Bertelloni S, Erba P, Zampollo E, Giuca MR. Pamidronate treatment stimulates the onset of recovery phase reducing fracture rate and skeletal deformities in patients with idiopathic juvenile osteoporosis: comparison with untreated patients. J Bone Miner Metab. 2013; 31:533–43.
Article24. Ward L, Tricco AC, Phuong P, Cranney A, Barrowman N, Gaboury I, et al. Bisphosphonate therapy for children and adolescents with secondary osteoporosis. Cochrane Database Syst Rev. 2007; (4):CD005324.
Article25. Plotkin H, Coughlin S, Kreikemeier R, Heldt K, Bruzoni M, Lerner G. Low doses of pamidronate to treat osteopenia in children with severe cerebral palsy: a pilot study. Dev Med Child Neurol. 2006; 48:709–12.
Article26. Lee YA, Lim JS, Shin CH, Yang SW. Pamidronate therapy in children and adolescents with osteoporosis. Horm Res. 2008; 70:27.27. Moon SJ, An YM, Kim SK, Kwon YS, Lee JE. The effect of low-dose intravenous bisphosphonate treatment on osteoporosis in children with quadriplegic cerebral palsy. Korean J Pediatr. 2017; 60:403–7.
Article28. Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004; 363:1377–85.
Article29. Swaminathan R. Biochemical markers of bone turnover. Clin Chim Acta. 2001; 313:95–105.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Management of Osteoporosis in Children and Adolescents
- Efficacy of pamidronate in children with low bone mineral density during and after chemotherapy for acute lymphoblastic leukemia and non-Hodgkin lymphoma
- Pamidronate Therapy in Children and Adolescents with Secondary Osteoporosis
- Effect of cyclic pamidronate administration on osteoporosis in children with β-thalassemia major: a single-center study
- Short-term Efficacy of Monthly Pamidronate Infusion in Patients with Osteogenesis Imperfecta