J Pathol Transl Med.
2021 May;55(3):192-201. 10.4132/jptm.2021.01.23.
Identification of PI3K-AKT signaling as the dominant altered pathway in intestinal type ampullary cancers through whole-exome sequencing
- Affiliations
-
- 1Departments of Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
- 2Departments of Surgical Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
- 3Departments of Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
- 4Department of Pathology & Lab Medicine, All India Institute of Medical Sciences, Raebareli, India
- KMID: 2515924
- DOI: http://doi.org/10.4132/jptm.2021.01.23
Abstract
- Background
The genetic landscape of intestinal (INT) and pancreatobiliary (PB) type ampullary cancer (AC) has been evolving with distinct as well as overlapping molecular profiles.
Methods
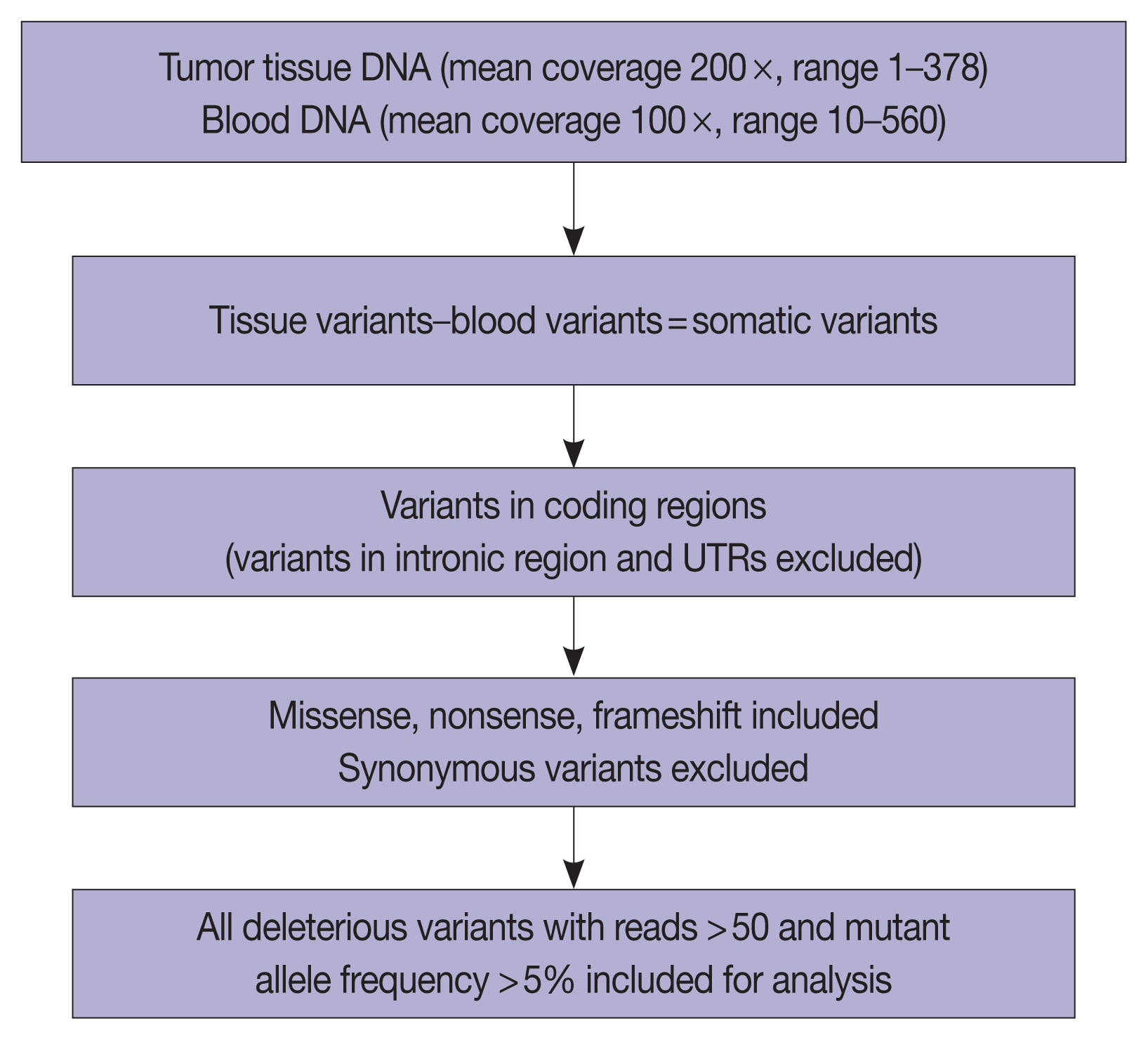
We performed whole-exome sequencing in 37 cases of AC to identify the targetable molecular profiles of INT and PB tumors. Paired tumor-normal sequencing was performed on the HiSeq 2500 Illumina platform.
Results
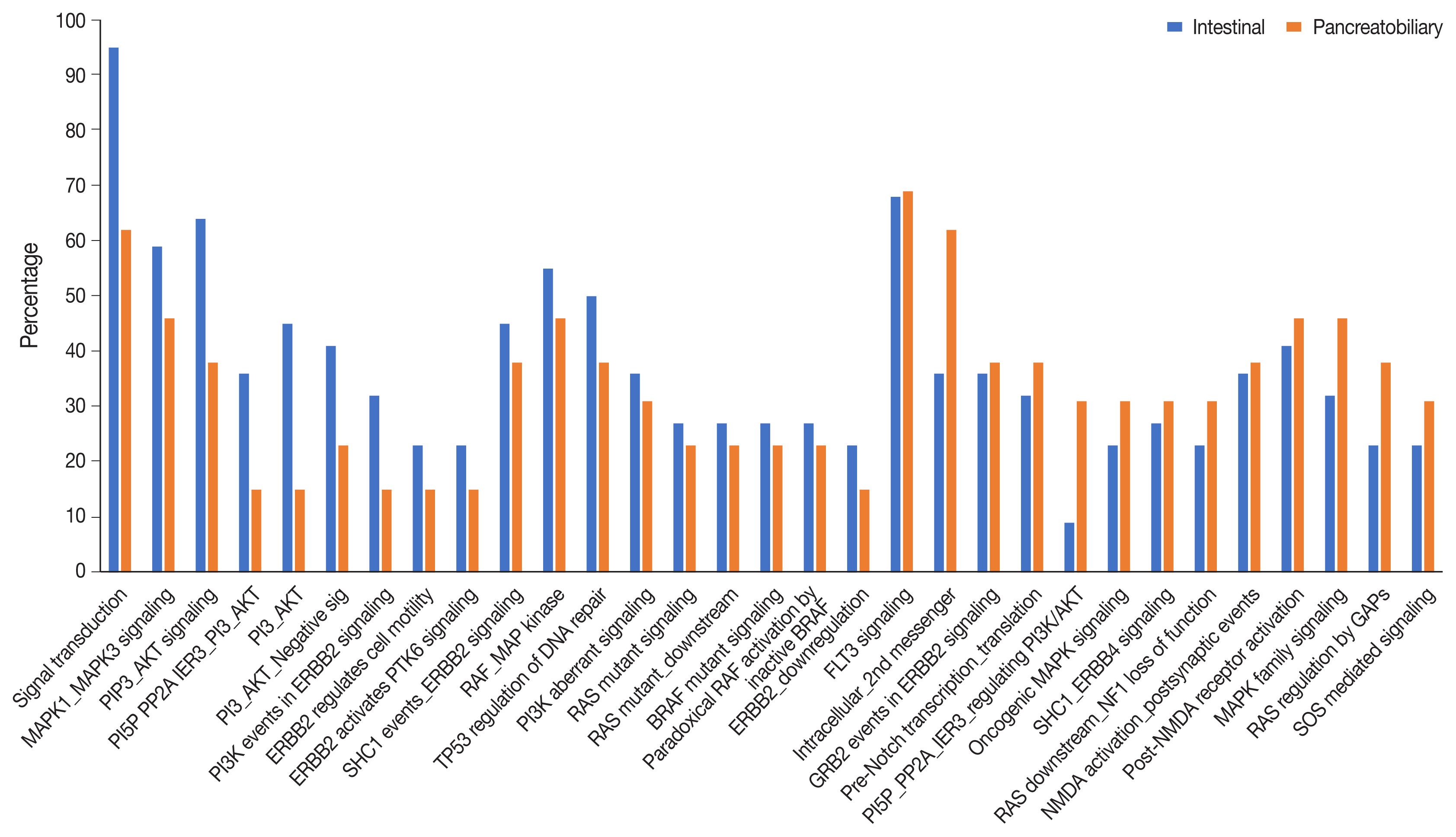
There were 22 INT, 13 PB, and two cases of mixed differentiation of AC that exhibited a total of 1,263 somatic variants in 112 genes (2–257 variants/case) with 183 somatic deleterious variants. INT showed variations in 78 genes (1–31/case), while PB showed variations in 51 genes (1–29/case). Targetable mutations involving one or more major pathways were found in 86.5% of all ACs. Mutations in APC, CTNNB1, SMAD4, KMT2, EPHA, ERBB, and Notch genes were more frequent in INT tumors, while chromatin remodeling complex mutations were frequent in PB tumors. In the major signaling pathways, the phosphoinositide 3-kinase (PI3)/AKT and RAS/mitogen-activated protein kinase (MAPK) pathways were significantly mutated in 70% of cases (82% INT, 46% PB, p = .023), with PI3/AKT mutation being more frequent in INT and RAS/MAPK in PB tumors. Tumor mutation burden was low in both differentiation types, with 1.6/Mb in INT and 0.8/Mb in PB types (p =.217).
Conclusions
The exome data suggest that INT types are genetically more unstable than PB and involve mutations in tumor suppressors, oncogenes, transcription factors, and chromatin remodeling genes. The spectra of the genetic profiles of INT and PB types suggested primary targeting of PI3/AKT in INT and RAS/RAF and PI3/AKT pathways in PB carcinomas.
Keyword
Figure
Cited by 1 articles
-
Histologic subtyping of ampullary carcinoma for targeted therapy
Seung-Mo Hong
J Pathol Transl Med. 2021;55(3):235-235. doi: 10.4132/jptm.2021.04.28.
Reference
-
References
1. Adsay V, Ohike N, Tajiri T, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012; 36:1592–608.2. Benhamiche AM, Jouve JL, Manfredi S, Prost P, Isambert N, Faivre J. Cancer of the ampulla of Vater: results of a 20-year population-based study. Eur J Gastroenterol Hepatol. 2000; 12:75–9.3. Demeure MJ, Craig DW, Sinari S, et al. Cancer of the ampulla of Vater: analysis of the whole genome sequence exposes a potential therapeutic vulnerability. Genome Med. 2012; 4:56.
Article4. Kim WS, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol. 2012; 105:266–72.
Article5. Colussi O, Voron T, Pozet A, et al. Prognostic score for recurrence after Whipple’s pancreaticoduodenectomy for ampullary carcinomas; results of an AGEO retrospective multicenter cohort. Eur J Surg Oncol. 2015; 41:520–6.
Article6. Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012; 308:147–56.7. Shoji H, Morizane C, Hiraoka N, et al. Twenty-six cases of advanced ampullary adenocarcinoma treated with systemic chemotherapy. Jpn J Clin Oncol. 2014; 44:324–30.
Article8. Kimura W, Futakawa N, Yamagata S, et al. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J Cancer Res. 1994; 85:161–6.
Article9. Albores-Saveedra J, Henson DE, Klimstra DS. Tumors of the gallbladder, extrahepatic bile ducts, and ampulla of Vater. Atlas of tumor pathology. 3rd series. 27. Washington, DC: Armed Forces Institute of Pathology;2000. p. 259–316.10. Kumari N, Prabha K, Singh RK, Baitha DK, Krishnani N. Intestinal and pancreatobiliary differentiation in periampullary carcinoma: the role of immunohistochemistry. Hum Pathol. 2013; 44:2213–9.
Article11. Yachida S, Wood LD, Suzuki M, et al. Genomic sequencing identifies ELF3 as a driver of ampullary carcinoma. Cancer Cell. 2016; 29:229–40.
Article12. Gingras MC, Covington KR, Chang DK, et al. Ampullary cancers harbor ELF3 tumor suppressor gene mutations and exhibit frequent WNT dysregulation. Cell Rep. 2016; 14:907–19.
Article13. Lundgren S, Hau SO, Elebro J, et al. Mutational landscape in resected periampullary adenocarcinoma: relationship with morphology and clinical outcome. JCO Precis Oncol. 2019; 3:PO1800323.
Article14. Perkins G, Svrcek M, Bouchet-Doumenq C, et al. Can we classify ampullary tumours better? Clinical, pathological and molecular features: results of an AGEO study. Br J Cancer. 2019; 120:697–702.
Article15. Overman MJ, Soifer HS, Schueneman AJ, et al. Performance and prognostic utility of the 92-gene assay in the molecular subclassification of ampullary adenocarcinoma. BMC Cancer. 2016; 16:668.
Article16. The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012; 487:330–7.17. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017; 32:185–203.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of PI3K/AKT Pathway and NADPH Oxidase 4 in Host ROS Manipulation by Toxoplasma gondii
- Developmental Exposure to Di-(2-ethylhexyl) Phthalate Induces Cerebellar Granule Cell Apoptosis via the PI3K/AKT Signaling Pathway
- Targeting the Phosphatidylinositol-3-kinase Pathway in Gastric Cancer: Can Omics Improve Outcomes?
- Curcumin targets vascular endothelial growth factor viaactivating the PI3K/Akt signaling pathway and improves brainhypoxic-ischemic injury in neonatal rats
- MicroRNA-21 promotes epithelial-mesenchymal transition and migration of human bronchial epithelial cells by targeting poly (ADP-ribose) polymerase-1 and activating PI3K/AKT signaling