Cancer Res Treat.
2021 Jan;53(1):252-260. 10.4143/crt.2020.275.
The Broad Variability in Dental Age Observed among Childhood Survivors Is Cancer Specific
- Affiliations
-
- 1Department of Pediatric Dentistry, Medical University of Lodz, Lodz, Poland
- 2Department of Pediatrics, Diabetology, Endocrinology and Nephrology, Medical University of Lodz, Lodz, Poland
- 3Department of Pediatrics, Oncology and Hematology, Medical University of Lodz, Lodz, Poland
- KMID: 2510667
- DOI: http://doi.org/10.4143/crt.2020.275
Abstract
- Purpose
The study aimed to assess the differences in dental maturation between childhood cancer survivors and healthy children.
Materials and Methods
Fifty-nine cancer patients including 16 (27.1%) girls and 43 (72.8%) boys, aged between 4 and 16 years, underwent dental and radiographic examinations. The mean duration of anticancer therapy was 16.8 months (range, 1 to 47 months), and 4.6 years (range, 8 to 123 months) had passed since the termination of disease. The control group consisted of 177 panoramic radiographs of age- and sex-matched healthy individuals. Dental age (DA) was estimated with Demirjian’s scale and delta age, i.e., DA–chronological age (CA), was used to compare groups.
Results
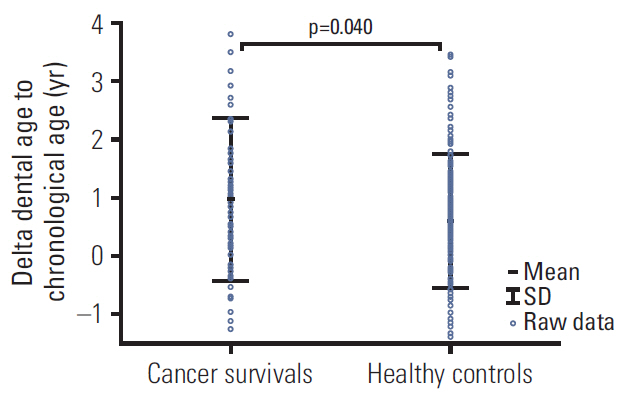
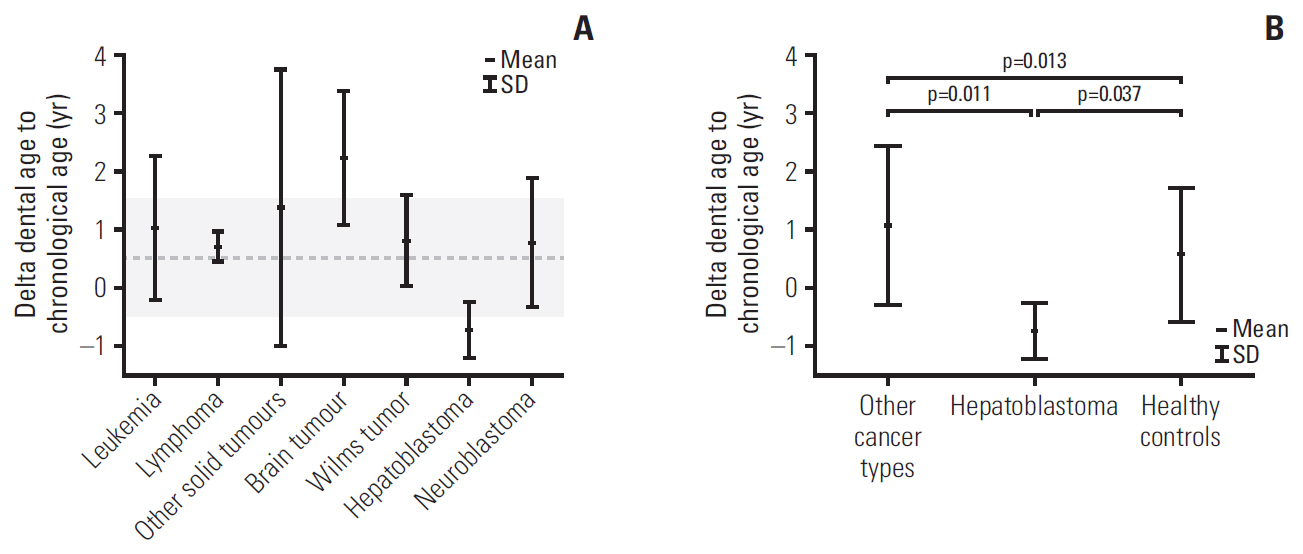
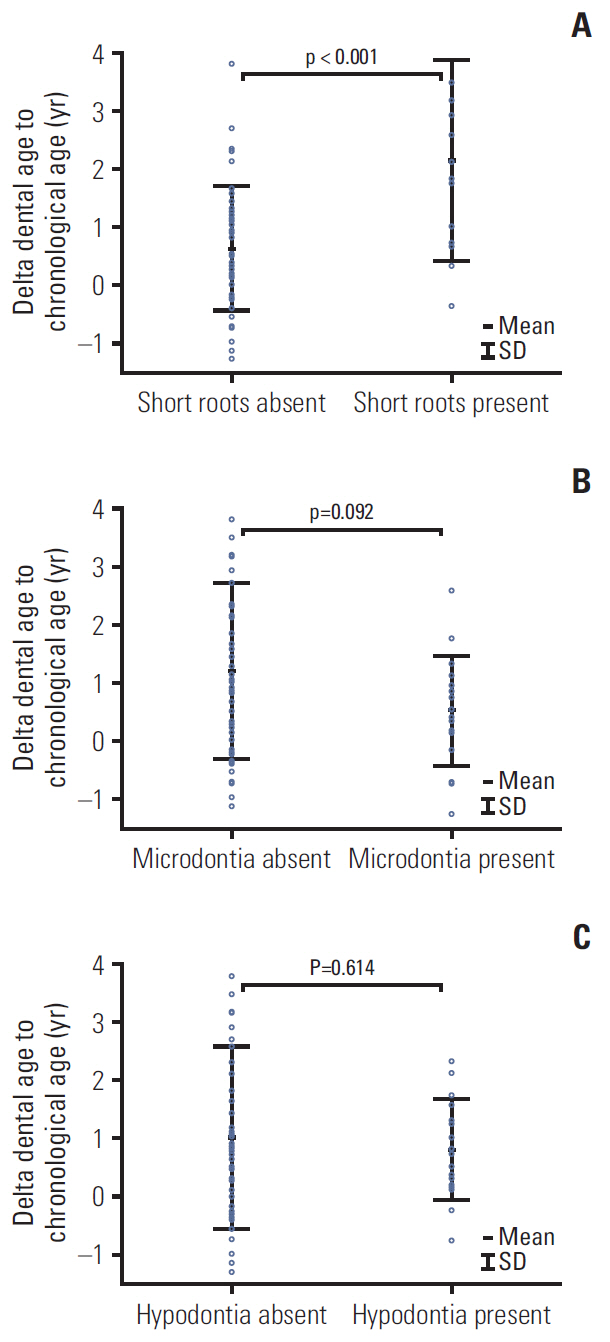
The DA of cancer survivors was accelerated by almost 1 year compared to their CA (9.9±3.1 vs. 8.9±2.8, p=0.040). The greatest difference was observed among patients with brain tumor: delta (DA–CA) was 2.2±1.1 years. Among all cancer patients, only children with familial adenomatous polyposis (FAP)-associated hepatoblastoma (HP) demonstrated delayed DA, with regard to both other cancer survivors (p=0.011) and healthy patients (p=0.037). All four patients with HP suffered from FAP, and three of them had documented adenomatous polyposis coli (APC) genes mutation. The DA of cancer patients having teeth with short roots was significantly greater than that of the cancer survivors without this anomaly (12.8±3.2 vs. 9.0±2.4, p < 0.001).
Conclusion
DA in children may be altered by cancer disease.
Figure
Reference
-
References
1. Zubowska M, Wyka K, Fendler W, Mlynarski W, Zalewska-Szewczyk B. Interleukin 18 as a marker of chronic nephropathy in children after anticancer treatment. Dis Markers. 2013; 35:811–8.
Article2. Kang CM, Hahn SM, Kim HS, Lyu CJ, Lee JH, Lee J, et al. Clinical risk factors influencing dental developmental disturbances in childhood cancer survivors. Cancer Res Treat. 2018; 50:926–35.
Article3. Proc P, Szczepanska J, Skiba A, Zubowska M, Fendler W, Mlynarski W. Dental anomalies as late sdverse rffect among young children treated for cancer. Cancer Res Treat. 2016; 48:658–67.4. Kawakami T, Nakamura Y, Karibe H. Cyclophosphamide-induced morphological changes in dental root development of ICR mice. PLoS One. 2015; 10:e0133256.
Article5. Kaste SC, Goodman P, Leisenring W, Stovall M, Hayashi RJ, Yeazel M, et al. Impact of radiation and chemotherapy on risk of dental abnormalities: a report from the Childhood Cancer Survivor Study. Cancer. 2009; 115:5817–27.6. Avery JK, Steele PF, Avery N. Oral development and histology. 3rd ed. Stuttgart: Thieme;2002.7. Demirjian A, Goldstein H, Tanner JM. A new system of dental age assessment. Hum Biol. 1973; 45:211–27.8. Holtta P, Alaluusua S, Saarinen-Pihkala UM, Peltola J, Hovi L. Agenesis and microdontia of permanent teeth as late adverse effects after stem cell transplantation in young children. Cancer. 2005; 103:181–90.
Article9. Holtta P, Hovi L, Saarinen-Pihkala UM, Peltola J, Alaluusua S. Disturbed root development of permanent teeth after pediatric stem cell transplantation. Dental root development after SCT. Cancer. 2005; 103:1484–93.10. Martin MB, Li CS, Rowland CC, Howard SC, Kaste SC. Correlation of bone age, dental age, and chronological age in survivors of childhood acute lymphoblastic leukaemia. Int J Paediatr Dent. 2008; 18:217–23.
Article11. van der Pas-van Voskuilen IG, Veerkamp JS, Raber-Durlacher JE, Bresters D, van Wijk AJ, Barasch A, et al. Long-term adverse effects of hematopoietic stem cell transplantation on dental development in children. Support Care Cancer. 2009; 17:1169–75.
Article12. Flores AP, Monti CF, Brunotto M. Dental and chronological age in children under oncological treatment. J Forensic Sci. 2015; 60:453–6.
Article13. Tomas LF, Monico LS, Tomas I, Varela-Patino P, Martin-Biedma B. The accuracy of estimating chronological age from Demirjian and Nolla methods in a Portuguese and Spanish sample. BMC Oral Health. 2014; 14:160.
Article14. Rozylo-Kalinowska I, Kiworkowa-Raczkowska E, Kalinowski P. Dental age in Central Poland. Forensic Sci Int. 2008; 174:207–16.15. Sobieska E, Fester A, Nieborak M, Zadurska M. Assessment of the dental age of children in the Polish population with comparison of the Demirjian and the Willems methods. Med Sci Monit. 2018; 24:8315–21.
Article16. Chudasama PN, Roberts GJ, Lucas VS. Dental age assessment (DAA): a study of a Caucasian population at the 13 year threshold. J Forensic Leg Med. 2012; 19:22–8.
Article17. Esan TA, Schepartz LA. The timing of permanent tooth development in a Black Southern African population using the Demirjian method. Int J Legal Med. 2019; 133:257–68.
Article18. Wang J, Bai X, Wang M, Zhou Z, Bian X, Qiu C, et al. Applicability and accuracy of Demirjian and Willems methods in a population of Eastern Chinese subadults. Forensic Sci Int. 2018; 292:90–6.
Article19. Jayaraman J, Roberts GJ. Comparison of dental maturation in Hong Kong Chinese and United Kingdom Caucasian populations. Forensic Sci Int. 2018; 292:61–70.
Article20. Chehab DA, Tanbonliong T, Peyser J, Udin R. Association between body mass index and dental age in Hispanic children. Gen Dent. 2017; 65:54–8.21. Hashim HA, Mansoor H, Mohamed MH. Assessment of skeletal age using hand-wrist radiographs following Bjork system. J Int Soc Prev Community Dent. 2018; 8:482–7.22. Ferrandez A, Carrascosa A, Audi L, Baguer L, Rueda C, Bosch-Castane J, et al. Longitudinal pubertal growth according to age at pubertal growth spurt onset: data from a Spanish study including 458 children (223 boys and 235 girls). J Pediatr Endocrinol Metab. 2009; 22:715–26.
Article23. Brinksma A, Roodbol PF, Sulkers E, Kamps WA, de Bont ES, Boot AM, et al. Changes in nutritional status in childhood cancer patients: a prospective cohort study. Clin Nutr. 2015; 34:66–73.
Article24. Paulsson L, Arvini S, Bergstrom N, Klingberg G, Lindh C. The impact of premature birth on dental maturation in the permanent dentition. Clin Oral Investig. 2019; 23:855–61.
Article25. Giardiello FM, Petersen GM, Brensinger JD, Luce MC, Cayouette MC, Bacon J, et al. Hepatoblastoma and APC gene mutation in familial adenomatous polyposis. Gut. 1996; 39:867–9.
Article26. Lubinsky M, Kantaputra PN. Syndromes with supernumerary teeth. Am J Med Genet A. 2016; 170:2611–6.
Article27. Basaran G, Erkan M. One of the rarest syndromes in dentistry: gardner syndrome. Eur J Dent. 2008; 2:208–12.
Article28. Ruiz-Mealin EV, Parekh S, Jones SP, Moles DR, Gill DS. Radiographic study of delayed tooth development in patients with dental agenesis. Am J Orthod Dentofacial Orthop. 2012; 141:307–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of Risk Factors for Dental Developmental Disorders in Pediatric Cancer Survivors
- Childhood Cancer Survivor's Services Needs for the Better Quality of Life
- Long-term follow-up study and long-term care of childhood cancer survivors
- Health-related Needs and Quality of Life in Childhood Cancer Survivors
- Late physical effects of childhood cancer survivors