Cancer Res Treat.
2021 Jan;53(1):233-242. 10.4143/crt.2020.159.
A Preoperative Nomogram for Predicting Chemoresistance to Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Squamous Carcinoma Treated with Radical Hysterectomy
- Affiliations
-
- 1Department of Gynecology Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- KMID: 2510665
- DOI: http://doi.org/10.4143/crt.2020.159
Abstract
- Purpose
This study aimed to investigate the factors associated with chemoresistance to neoadjuvant chemotherapy (NACT) followed by radical hysterectomy (RH) and construct a nomogram to predict the chemoresistance in patients with locally advanced cervical squamous carcinoma (LACSC).
Materials and Methods
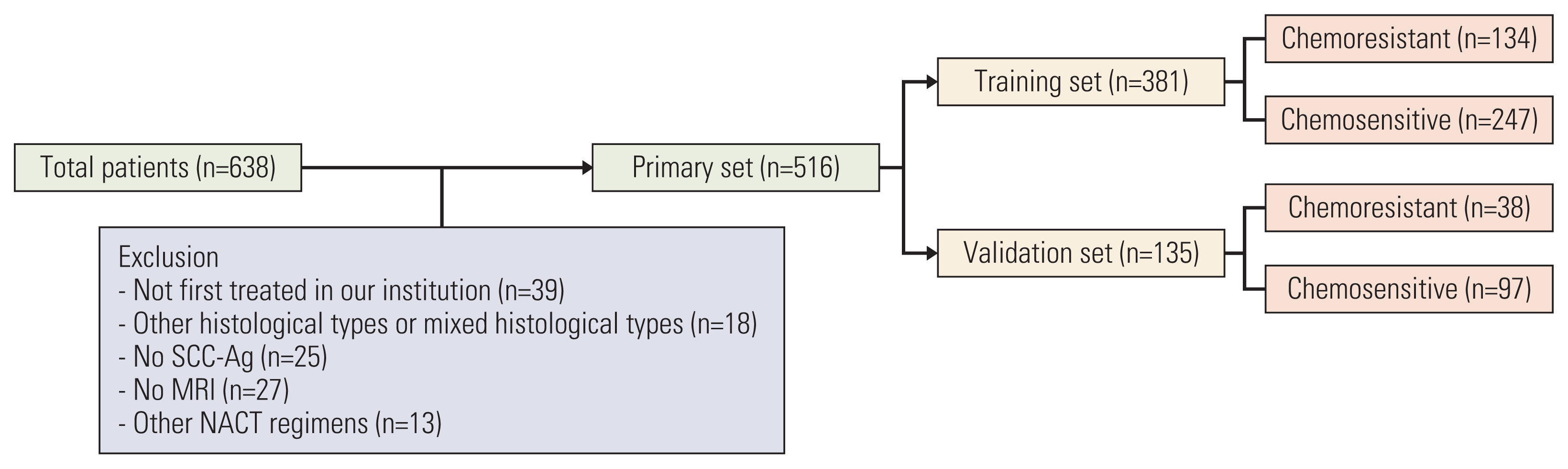
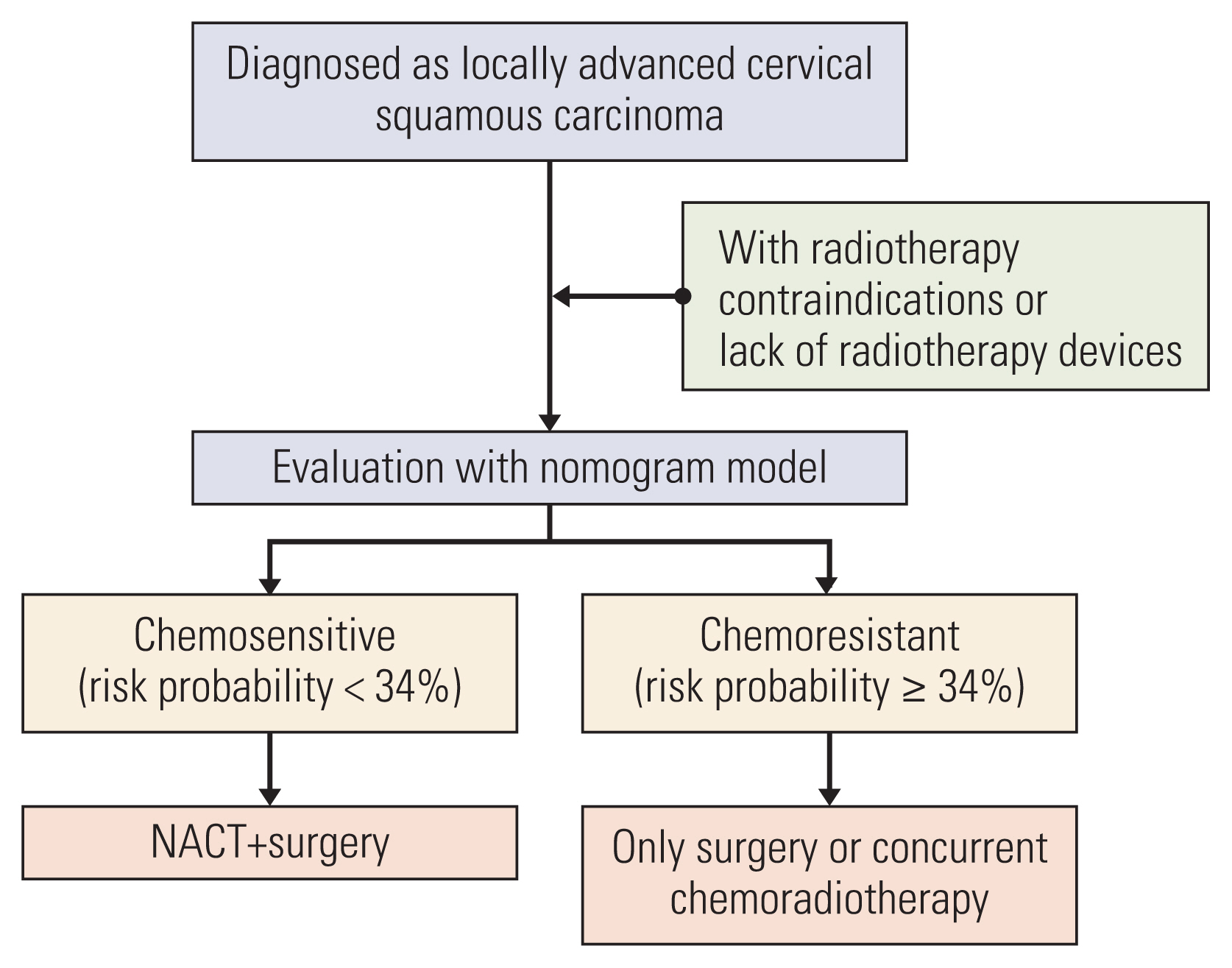
This retrospective study included 516 patients with International Federation of Gynecology and Obstetrics (2003) stage IB2 and IIA2 cervical cancer treated with NACT and RH between 2007 and 2017. Clinicopathologic data were collected, and patients were assigned to training (n=381) and validation (n=135) sets. Univariate and multivariate analyses were performed to analyze factors associated with chemoresistance to NACT. A nomogram was built using the multivariate logistic regression analysis results. We evaluated the discriminative ability and accuracy of the model using a concordance index and a calibration curve. The predictive probability of chemoresistance to NACT was defined as > 34%.
Results
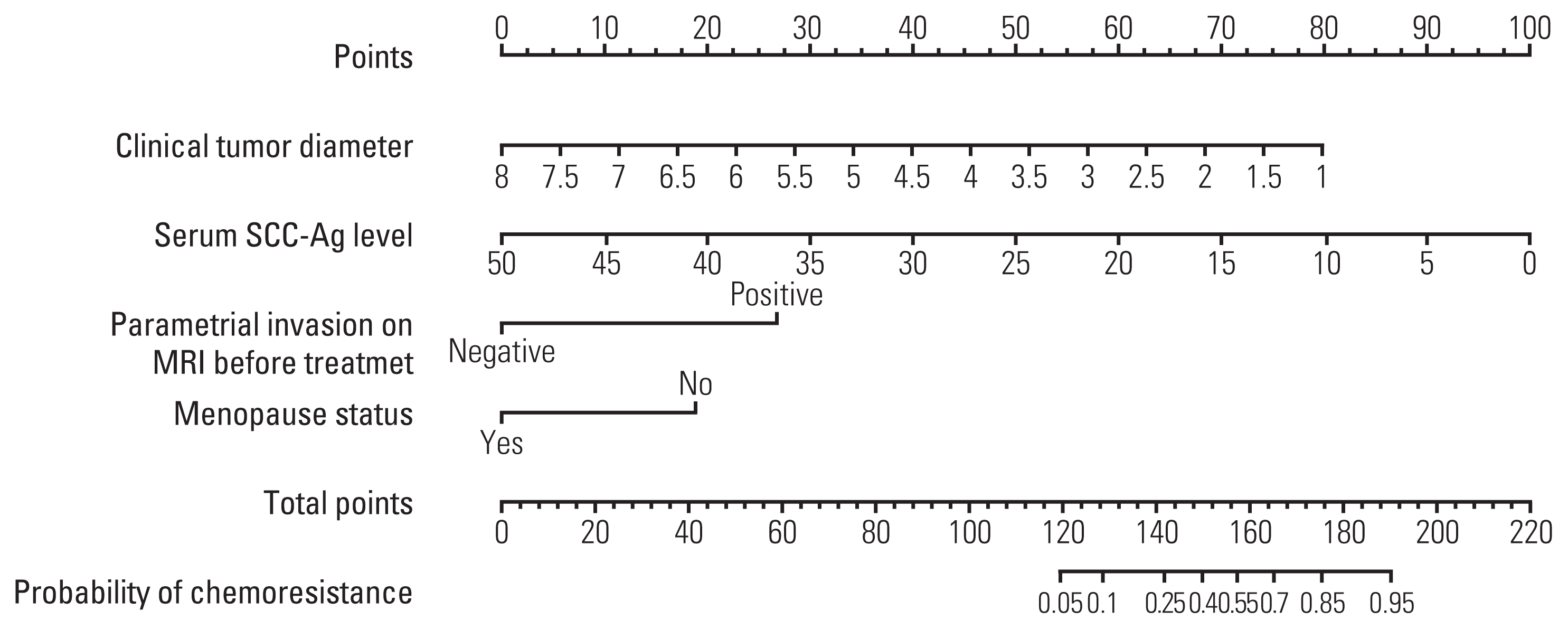
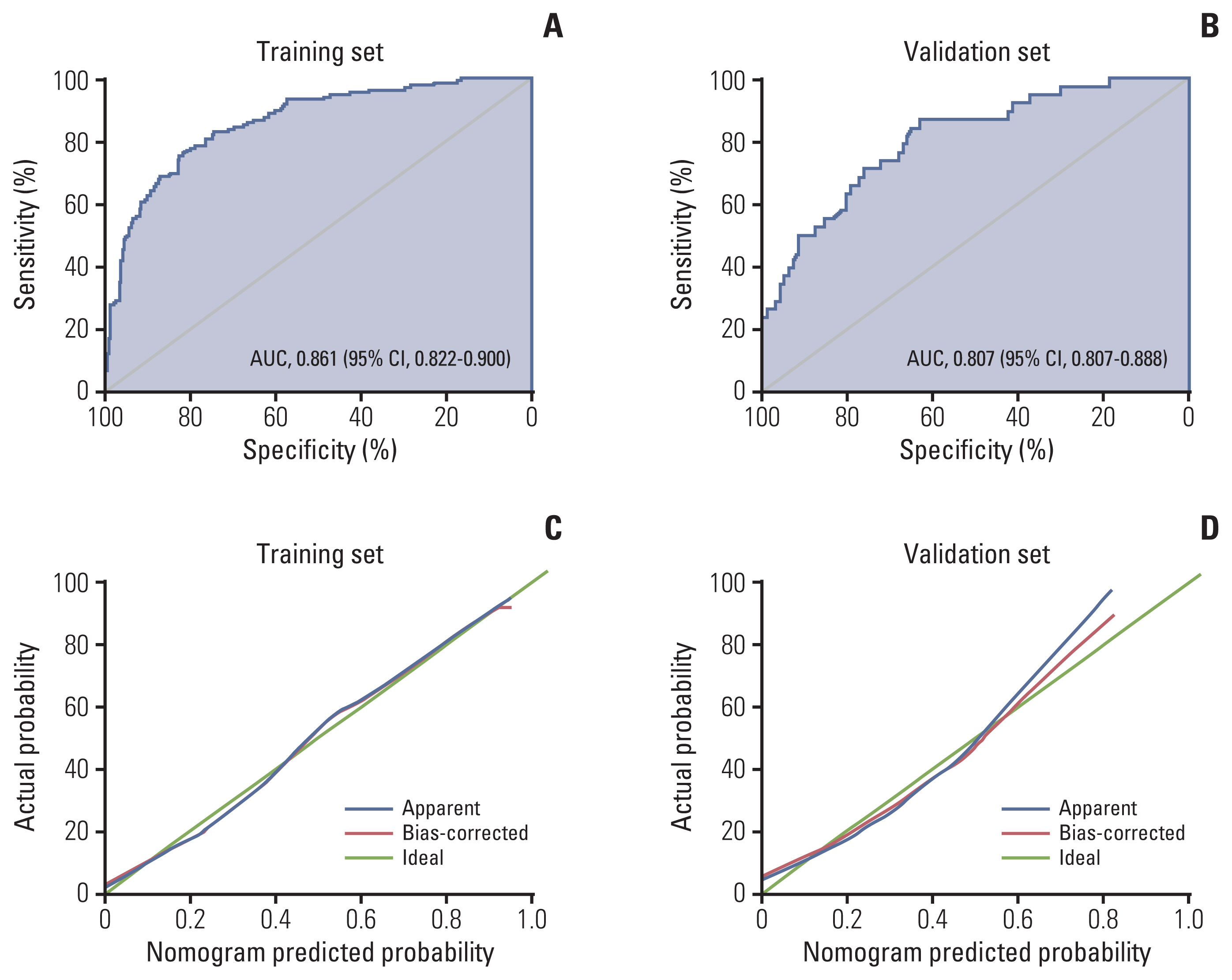
Multivariate analysis confirmed menopausal status, clinical tumor diameter, serum squamous cell carcinoma antigen level, and parametrial invasion on magnetic resonance imaging before treatment as independent prognostic factors associated with chemoresistance to NACT. The concordance indices of the nomogram for training and validation sets were 0.861 (95% confidence interval [CI], 0.822 to 0.900) and 0.807 (95% CI, 0.807 to 0.888), respectively. Calibration plots revealed a good fit between the modelpredicted probabilities and actual probabilities (Hosmer-Lemeshow test, p=0.597). Furthermore, grouping based on the nomogram was associated with progression-free survival.
Conclusion
We developed a nomogram for predicting chemoresistance in LACSC patients treated with RH. This nomogram can help physicians make clinical decisions regarding primary management and postoperative follow-up of the patients.
Figure
Reference
-
References
1. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018; 143(Suppl 2):22–36.
Article2. He D, Duan C, Chen J, Lai L, Chen J, Chen D. The safety and efficacy of the preoperative neoadjuvant chemotherapy for patients with cervical cancer: a systematic review and meta analysis. Int J Clin Exp Med. 2015; 8:14693–700.3. Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer. 2003; 39:2470–86.4. Hsieh HY, Huang JW, Lu CH, Lin JC, Wang L. Definite chemoradiotherapy is a competent treatment option in FIGO stage IB2 cervical cancer compared with radical surgery +/− neoadjuvant chemotherapy. J Formos Med Assoc. 2019; 118:99–108.
Article5. Katsumata N, Yoshikawa H, Kobayashi H, Saito T, Kuzuya K, Nakanishi T, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan Clinical Oncology Group trial (JCOG 0102). Br J Cancer. 2013; 108:1957–63.
Article6. Marita A, Ordeanu C, Rancea A, Nicolae T, Nagy VM. Long-term survival following neoadjuvant chemotherapy and concomitant radiochemotherapy in locally advanced cervical cancer: results of the Oncology Institute “Prof. Dr. Ion Chiricuta” experience. J Med Life. 2018; 11:42–50.7. Chen H, Liang C, Zhang L, Huang S, Wu X. Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) cervical cancer: randomized study. Gynecol Oncol. 2008; 110:308–15.8. Li J, Wu MF, Lu HW, Chen Q, Lin ZQ, Wang LJ. Pretreatment serum lactate dehydrogenase is an independent prognostic factor for patients receiving neoadjuvant chemotherapy for locally advanced cervical cancer. Cancer Med. 2016; 5:1863–72.
Article9. Yin M, Hou Y, Zhang T, Cui C, Zhou X, Sun F, et al. Evaluation of chemotherapy response with serum squamous cell carcinoma antigen level in cervical cancer patients: a prospective cohort study. PLoS One. 2013; 8:e54969.
Article10. Sun C, Tian X, Liu Z, Li W, Li P, Chen J, et al. Radiomic analysis for pretreatment prediction of response to neoadjuvant chemotherapy in locally advanced cervical cancer: a multicentre study. EBioMedicine. 2019; 46:160–9.
Article11. Mayr NA, Taoka T, Yuh WT, Denning LM, Zhen WK, Paulino AC, et al. Method and timing of tumor volume measurement for outcome prediction in cervical cancer using magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2002; 52:14–22.
Article12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article13. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008; 26:1364–70.
Article14. Scambia G, Benedetti Panici P, Foti E, Amoroso M, Salerno G, Ferrandina G, et al. Squamous cell carcinoma antigen: prognostic significance and role in the monitoring of neoadjuvant chemotherapy response in cervical cancer. J Clin Oncol. 1994; 12:2309–16.
Article15. Wang T, Gao T, Yang J, Yan X, Wang Y, Zhou X, et al. Preoperative prediction of pelvic lymph nodes metastasis in early-stage cervical cancer using radiomics nomogram developed based on T2-weighted MRI and diffusion-weighted imaging. Eur J Radiol. 2019; 114:128–35.
Article16. Poujade O, Morice P, Rouzier R, Madelenat P, Lecuru F, Muray JM, et al. Pathologic response rate after concomitant neo-adjuvant radiotherapy and chemotherapy for adenocarcinoma of the uterine cervix: a retrospective multicentric study. Int J Gynecol Cancer. 2010; 20:815–20.
Article17. Del Prete S, Caraglia M, Luce A, Montella L, Galizia G, Sperlongano P, et al. Clinical and pathological factors predictive of response to neoadjuvant chemotherapy in breast cancer: a single center experience. Oncol Lett. 2019; 18:3873–9.
Article18. Ouldamer L, Chas M, Arbion F, Body G, Cirier J, Ballester M, et al. Risk scoring system for predicting axillary response after neoadjuvant chemotherapy in initially node-positive women with breast cancer. Surg Oncol. 2018; 27:158–65.
Article19. Kong TW, Kim J, Son JH, Kang SW, Paek J, Chun M, et al. Preoperative nomogram for prediction of microscopic parametrial infiltration in patients with FIGO stage IB cervical cancer treated with radical hysterectomy. Gynecol Oncol. 2016; 142:109–14.
Article20. Resende U, Cabello C, Oliveira Botelho Ramalho S, Zeferino LC. Predictors of pathological complete response in women with clinical complete response to neoadjuvant chemotherapy in breast carcinoma. Oncology. 2018; 95:229–38.
Article21. Hug V, Thames HD Jr, Polyzos A, Johnston D. Chemoresistance is not a cause of the apparent failure of adjuvant chemotherapy in postmenopausal women. Eur J Cancer Clin Oncol. 1988; 24:713–8.
Article22. Hricak H, Lacey CG, Sandles LG, Chang YC, Winkler ML, Stern JL. Invasive cervical carcinoma: comparison of MR imaging and surgical findings. Radiology. 1988; 166:623–31.
Article23. Park JS, Jeon EK, Chun SH, Won HS, Lee A, Hur SY, et al. ERCC1 (excision repair cross-complementation group 1) expression as a predictor for response of neoadjuvant chemotherapy for FIGO stage 2B uterine cervix cancer. Gynecol Oncol. 2011; 120:275–9.
Article24. Rein DT, Kurbacher CM. The role of chemotherapy in invasive cancer of the cervix uteri: current standards and future prospects. Anticancer Drugs. 2001; 12:787–95.
Article25. Jiapaer S, Furuta T, Tanaka S, Kitabayashi T, Nakada M. Potential strategies overcoming the temozolomide resistance for glioblastoma. Neurol Med Chir (Tokyo). 2018; 58:405–21.
Article26. Fu J, Wang W, Wang Y, Liu C, Wang P. The role of squamous cell carcinoma antigen (SCC Ag) in outcome prediction after concurrent chemoradiotherapy and treatment decisions for patients with cervical cancer. Radiat Oncol. 2019; 14:146.
Article27. Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005; 4:307–20.
Article28. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014; 25:2677–81.
Article29. Lapresa M, Parma G, Portuesi R, Colombo N. Neoadjuvant chemotherapy in cervical cancer: an update. Expert Rev Anticancer Ther. 2015; 15:1171–81.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Survival Nomograms after Curative Neoadjuvant Chemotherapy and Radical Surgery for Stage IB2-IIIB Cervical Cancer
- Comparative study of neoadjuvant chemotherapy before radical hysterectomy and radical surgery alone in stage IB2-IIA bulky cervical cancer
- Survival Benefits of Neoadjuvant Chemotherapy Followed by Radical Surgery versus Radiotherapy in Locally Advanced Chemoresistant Cervical Cancer
- The Study for Usefulness of Overexpression of bcl-2 , p53 Gene and Apoptosis as a Response Predictor to Neoadjuvant Chemotherapy in Cervical Cancer
- Recent Management of FIGO stage IB2 Cervical Cancer