Diabetes Metab J.
2019 Jun;43(3):368-376. 10.4093/dmj.2018.0066.
Serum R-Spondin 1 Is a New Surrogate Marker for Obesity and Insulin Resistance
- Affiliations
-
- 1Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea. bonjeong@cnu.ac.kr, kimhj43@cnuh.co.kr
- KMID: 2454063
- DOI: http://doi.org/10.4093/dmj.2018.0066
Abstract
- BACKGROUND
Recent in vivo studies indicated that R-spondin 1 (RSPO1) regulates food intake and increases insulin secretion, but its role in humans remains unknown. This study investigated the association between serum levels of RSPO1 and diverse metabolic parameters in humans.
METHODS
The study population consisted of 43 subjects with newly diagnosed diabetes mellitus, and 79 non-diabetic participants. Serum levels of RSPO1 were measured using the enzyme-linked immunosorbent assay. The relationships between circulating RSPO1 and diverse metabolic parameters were analyzed.
RESULTS
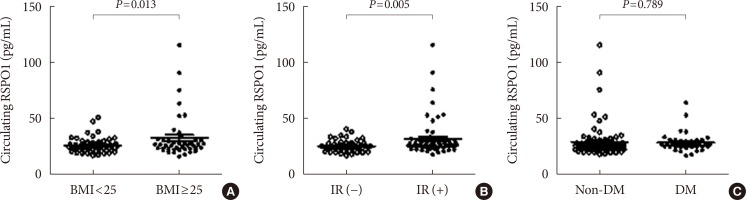
Circulating RSPO1 levels increased to a greater extent in the obese group than in the lean group. Moreover, serum levels of RSPO1 were higher in the insulin-resistant group than in the insulin-sensitive group. Serum levels of RSPO1 were significantly correlated with a range of metabolic parameters including body mass index, fasting C-peptide, homeostasis model assessment of insulin resistance index, and lipid profile. Moreover, levels were significantly associated with insulin resistance and obesity in non-diabetic subjects.
CONCLUSION
This study demonstrated the association between serum levels of RSPO1 and a range of metabolic parameters in humans. Serum levels of RSPO1 are significantly related to obesity and insulin resistance, although the precise mechanisms remain unknown.
Keyword
MeSH Terms
Figure
Reference
-
1. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004; 20:781–810. PMID: 15473860.
Article2. Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010; 137:1407–1420. PMID: 20388652.
Article3. Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003; 4:407–418. PMID: 12636921.4. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006; 127:469–480. PMID: 17081971.5. Saxena R, Gianniny L, Burtt NP, Lyssenko V, Giuducci C, Sjogren M, Florez JC, Almgren P, Isomaa B, Orho-Melander M, Lindblad U, Daly MJ, Tuomi T, Hirschhorn JN, Ardlie KG, Groop LC, Altshuler D. Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes. 2006; 55:2890–2895. PMID: 17003358.
Article6. Jin T, Liu L. The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus. Mol Endocrinol. 2008; 22:2383–2392. PMID: 18599616.7. Wright WS, Longo KA, Dolinsky VW, Gerin I, Kang S, Bennett CN, Chiang SH, Prestwich TC, Gress C, Burant CF, Susulic VS, MacDougald OA. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007; 56:295–303. PMID: 17259372.
Article8. Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, Opp MR, MacDougald OA. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004; 279:35503–35509. PMID: 15190075.
Article9. Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A, Tanaka Y, Babazono T, Matsuda M, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakamura Y, Maeda S. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am J Hum Genet. 2004; 75:832–843. PMID: 15386214.
Article10. Fuster JJ, Zuriaga MA, Ngo DT, Farb MG, Aprahamian T, Yamaguchi TP, Gokce N, Walsh K. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2015; 64:1235–1248. PMID: 25352637.
Article11. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012; 149:1192–1205. PMID: 22682243.
Article12. Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 2011; 12:1055–1061. PMID: 21909076.
Article13. de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012; 13:242. PMID: 22439850.
Article14. Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2011; 108:11452–11457. PMID: 21693646.15. Li JY, Chai B, Zhang W, Fritze DM, Zhang C, Mulholland MW. LGR4 and its ligands, R-spondin 1 and R-spondin 3, regulate food intake in the hypothalamus of male rats. Endocrinology. 2014; 155:429–440. PMID: 24280058.
Article16. Wong VS, Yeung A, Schultz W, Brubaker PL. R-spondin-1 is a novel beta-cell growth factor and insulin secretagogue. J Biol Chem. 2010; 285:21292–21302. PMID: 20442404.17. Wang J, Liu R, Wang F, Hong J, Li X, Chen M, Ke Y, Zhang X, Ma Q, Wang R, Shi J, Cui B, Gu W, Zhang Y, Zhang Z, Wang W, Xia X, Liu M, Ning G. Ablation of LGR4 promotes energy expenditure by driving white-to-brown fat switch. Nat Cell Biol. 2013; 15:1455–1463. PMID: 24212090.
Article18. Shin MY, Kim JM, Kang YE, Kim MK, Joung KH, Lee JH, Kim KS, Kim HJ, Ku BJ, Shong M. Association between growth differentiation factor 15 (GDF15) and cardiovascular risk in patients with newly diagnosed type 2 diabetes mellitus. J Korean Med Sci. 2016; 31:1413–1418. PMID: 27510384.
Article19. Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, Ryu MJ, Ko YB, Lee MA, Lee J, Ku BJ, Shong M, Lee KH, Kim HJ. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016; 11:e0154003. PMID: 27101398.
Article20. Kang YE, Choung S, Lee JH, Kim HJ, Ku BJ. The role of circulating Slit2, the one of the newly batokines, in human diabetes mellitus. Endocrinol Metab (Seoul). 2017; 32:383–388. PMID: 28956369.
Article21. Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet. 2005; 94:1–12. PMID: 16145245.
Article22. American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015; 38(Suppl):S8–S16.23. Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A, Ringnalda F, Begthel H, Hamer K, Mulder J, van Es JH, de Koning E, Vries RG, Heimberg H, Clevers H. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013; 32:2708–2721. PMID: 24045232.
Article24. Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013; 340:1190–1194. PMID: 23744940.
Article25. Welters HJ, Kulkarni RN. Wnt signaling: relevance to beta-cell biology and diabetes. Trends Endocrinol Metab. 2008; 19:349–355. PMID: 18926717.26. Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005; 309:1256–1259. PMID: 16109882.
Article27. Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014; 346:1248012. PMID: 25278615.
Article28. Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009; 20:16–24. PMID: 19008118.
Article29. Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002; 277:30998–31004. PMID: 12055200.
Article30. Prestwich TC, Macdougald OA. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007; 19:612–617. PMID: 17997088.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin Resistance and Insulin Resistance Syndrome
- Triglyceride Is a Useful Surrogate Marker for Insulin Resistance in Korean Women with Polycystic Ovary Syndrome
- Surrogate Measures of Insulin Resistance in an Apparently Healthy Population: a Simpler and Easier, yet Reliable Index
- Effects of Insulin Level on Dyslipidemia in Children with Simple Obesity
- Role of Interleukin-6 in Human Obesity and Insulin Resistance