Investig Clin Urol.
2016 Nov;57(6):377-383. 10.4111/icu.2016.57.6.377.
Secondary and tertiary treatments for multiple sclerosis patients with urinary symptoms
- Affiliations
-
- 1Department of Urology, University of Michigan, Ann Arbor, MI, USA. jstoffel@med.umich.edu
- KMID: 2451396
- DOI: http://doi.org/10.4111/icu.2016.57.6.377
Abstract
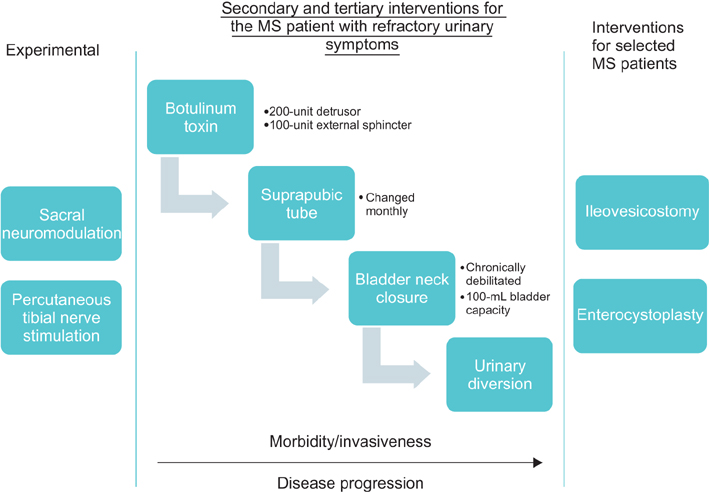
- Multiple sclerosis patients with refractory urinary symptoms after treatment with behavioral therapy and medications still have treatment options. Prior to starting treatments, baseline symptoms should be assessed and treatment goals thoroughly discussed. Catheterization, botulinum toxin, and reconstructive surgery all can play a role in improving both safety and quality of life for these patients. Newer modalities, such as neuromodulation, may also have an increasing role in the future as more data develop regarding efficacy. Risks need to be weighed against any perceived benefit and disease status before more aggressive therapy is initiated.
MeSH Terms
Figure
Reference
-
1. Rubin SM. Management of multiple sclerosis: an overview. Dis Mon. 2013; 59:253–260.2. Litwiller SE, Frohman EM, Zimmern PE. Multiple sclerosis and the urologist. J Urol. 1999; 161:743–757.3. Compston A, Coles A. Multiple sclerosis. Lancet. 2002; 359:1221–1231.4. Sadiq A, Brucker BM. Management of neurogenic lower urinary tract dysfunction in multiple sclerosis patients. Curr Urol Rep. 2015; 16:44.5. Mahajan ST, Patel PB, Marrie RA. Under treatment of overactive bladder symptoms in patients with multiple sclerosis: an ancillary analysis of the NARCOMS Patient Registry. J Urol. 2010; 183:1432–1437.6. Khalaf KM, Coyne KS, Globe DR, Armstrong EP, Malone DC, Burks J. Lower urinary tract symptom prevalence and management among patients with multiple sclerosis. Int J MS Care. 2015; 17:14–25.7. Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008; 9:453–466.8. Araki I, Matsui M, Ozawa K, Takeda M, Kuno S. Relationship of bladder dysfunction to lesion site in multiple sclerosis. J Urol. 2003; 169:1384–1387.9. Weissbart SJ, Pechersky D, Malykhina A, Bavaria T, Parrillo L, Arya LA, et al. The impact of pontine disease on lower urinary tract symptoms in patients with multiple sclerosis. Neurourol Urodyn. 2016; 01. 06. [Epub]. DOI: 10.1002/nau.22953.10. Patel DP, Elliott SP, Stoffel JT, Brant WO, Hotaling JM, Myers JB. Patient reported outcomes measures in neurogenic bladder and bowel: a systematic review of the current literature. Neurourol Urodyn. 2016; 35:8–14.11. Burks J, Chancellor M, Bates D, Denys P, Macdiarmid S, Nitti V, et al. Development and validation of the actionable bladder symptom screening tool for multiple sclerosis patients. Int J MS Care. 2013; 15:182–192.12. Welk B, Morrow S, Madarasz W, Baverstock R, Macnab J, Sequeira K. The validity and reliability of the neurogenic bladder symptom score. J Urol. 2014; 192:452–457.13. Gaspari M, Roveda G, Scandellari C, Stecchi S. An expert system for the evaluation of EDSS in multiple sclerosis. Artif Intell Med. 2002; 25:187–210.14. Fowler CJ, Panicker JN, Drake M, Harris C, Harrison SC, Kirby M, et al. A UK consensus on the management of the bladder in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009; 80:470–477.15. Stoffel JT. Contemporary management of the neurogenic bladder for multiple sclerosis patients. Urol Clin North Am. 2010; 37:547–557.16. Fletcher SG, Dillon BE, Gilchrist AS, Haverkorn RM, Yan J, Frohman EM, et al. Renal deterioration in multiple sclerosis patients with neurovesical dysfunction. Mult Scler. 2013; 19:1169–1174.17. de Sèze M, Ruffion A, Denys P, Joseph PA, Perrouin-Verbe B. GENULF. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler. 2007; 13:915–928.18. Nicholas RS, Friede T, Hollis S, Young CA. Anticholinergics for urinary symptoms in multiple sclerosis. Cochrane Database Syst Rev. 2009; (1):CD004193.19. Zahariou A, Karamouti M, Karagiannis G, Papaioannou P. Maximal bladder capacity is a positive predictor of response to desmopressin treatment in patients with MS and nocturia. Int Urol Nephrol. 2008; 40:65–69.20. Mahajan ST, Frasure HE, Marrie RA. The prevalence of urinary catheterization in women and men with multiple sclerosis. J Spinal Cord Med. 2013; 36:632–637.21. Vahter L, Zopp I, Kreegipuu M, Kool P, Talvik T, Gross-Paju K. Clean intermittent self-catheterization in persons with multiple sclerosis: the influence of cognitive dysfunction. Mult Scler. 2009; 15:379–384.22. Wyndaele JJ. Self-intermittent catheterization in multiple sclerosis. Ann Phys Rehabil Med. 2014; 57:315–320.23. Bolinger R, Engberg S. Barriers, complications, adherence, and self-reported quality of life for people using clean intermittent catheterization. J Wound Ostomy Continence Nurs. 2013; 40:83–89.24. James R, Frasure HE, Mahajan ST. Urinary catheterization may not adversely impact quality of life in multiple sclerosis patients. ISRN Neurol. 2014; 2014:167030.25. Stoffel JT, McGuire EJ. Outcome of urethral closure in patients with neurologic impairment and complete urethral destruction. Neurourol Urodyn. 2006; 25:19–22.26. Gobbi C, Digesu GA, Khullar V, El Neil S, Caccia G, Zecca C. Percutaneous posterior tibial nerve stimulation as an effective treatment of refractory lower urinary tract symptoms in patients with multiple sclerosis: preliminary data from a multicentre, prospective, open label trial. Mult Scler. 2011; 17:1514–1519.27. Canbaz Kabay S, Kabay S, Mestan E, Cetiner M, Ayas S, Sevim M, et al. Long term sustained therapeutic effects of percutaneous posterior tibial nerve stimulation treatment of neurogenic overactive bladder in multiple sclerosis patients: 12-months results. Neurourol Urodyn. 2015; 09. 09. [Epub]. DOI: 10.1002/nau.22868.28. Engeler DS, Meyer D, Abt D, Müller S, Schmid HP. Sacral neuromodulation for the treatment of neurogenic lower urinary tract dysfunction caused by multiple sclerosis: a single-centre prospective series. BMC Urol. 2015; 15:105.29. Ginsberg D, Gousse A, Keppenne V, Sievert KD, Thompson C, Lam W, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012; 187:2131–2139.30. Sussman D, Patel V, Del Popolo G, Lam W, Globe D, Pommerville P. Treatment satisfaction and improvement in health-related quality of life with onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity. Neurourol Urodyn. 2013; 32:242–249.31. Rovner E, Kohan A, Chartier-Kastler E, Jünemann KP, Del Popolo G, Herschorn S, et al. Long-term efficacy and safety of onabotulinumtoxina in patients with neurogenic detrusor overactivity who completed 4 years of treatment. J Urol. 2016; 196:801–808.32. Cruz F, Herschorn S, Aliotta P, Brin M, Thompson C, Lam W, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2011; 60:742–750.33. Deffontaines-Rufin S, Weil M, Verollet D, Peyrat L, Amarenco G. Botulinum toxin A for the treatment of neurogenic detrusor overactivity in multiple sclerosis patients. Int Braz J Urol. 2011; 37:642–648.34. Stoffel JT. Detrusor sphincter dyssynergia: a review of physiology, diagnosis, and treatment strategies. Transl Androl Urol. 2016; 5:127–135.35. Schurch B, de Sèze M, Denys P, Chartier-Kastler E, Haab F, Everaert K, et al. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005; 174:196–200.36. Kuo HC. Therapeutic outcome and quality of life between urethral and detrusor botulinum toxin treatment for patients with spinal cord lesions and detrusor sphincter dyssynergia. Int J Clin Pract. 2013; 67:1044–1049.37. Ginger VA, Miller JL, Yang CC. Bladder neck closure and suprapubic tube placement in a debilitated patient population. Neurourol Urodyn. 2010; 29:382–386.38. Colli J, Lloyd LK. Bladder neck closure and suprapubic catheter placement as definitive management of neurogenic bladder. J Spinal Cord Med. 2011; 34:273–277.39. Flood HD, Malhotra SJ, O'Connell HE, Ritchey MJ, Bloom DA, McGuire EJ. Long-term results and complications using augmentation cystoplasty in reconstructive urology. Neurourol Urodyn. 1995; 14:297–309.40. Khavari R, Fletcher SG, Liu J, Boone TB. A modification to augmentation cystoplasty with catheterizable stoma for neurogenic patients: technique and long-term results. Urology. 2012; 80:460–464.41. Gould JJ, Stoffel JT. Robotic enterocystoplasty: technique and early outcomes. J Endourol. 2011; 25:91–95.42. DeLong J, Tighiouart H, Stoffel J. Urinary diversion/reconstruction for cases of catheter intolerant secondary progressive multiple sclerosis with refractory urinary symptoms. J Urol. 2011; 185:2201–2206.43. Guillotreau J, Panicker JN, Castel-Lacanal E, Viala F, Roumiguié M, Malavaud B, et al. Prospective evaluation of laparoscopic assisted cystectomy and ileal conduit in advanced multiple sclerosis. Urology. 2012; 80:852–857.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Managing Urological Disorders in Multiple Sclerosis Patients: A Review of Available and Emerging Therapies
- Manifestations Like Multiple Sclerosis in a Paitent with Rheumatoid Arthritis and Sjogren's Syndrome
- Specificity of Lower Urinary Tract Symptoms in Neuromyelitis Optica in Comparison With Multiple Sclerosis Patients
- Stress Urinary Incontinence in Women With Multiple Sclerosis
- Lower Urinary Tract Symptoms in Elderly Population With Multiple Sclerosis