Yonsei Med J.
2015 May;56(3):726-736. 10.3349/ymj.2015.56.3.726.
A Network Analysis of 15O-H2O PET Reveals Deep Brain Stimulation Effects on Brain Network of Parkinson's Disease
- Affiliations
-
- 1Department of Nuclear Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 2BK21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. jchang@yuhs.ac
- 3Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2450347
- DOI: http://doi.org/10.3349/ymj.2015.56.3.726
Abstract
- PURPOSE
As Parkinson's disease (PD) can be considered a network abnormality, the effects of deep brain stimulation (DBS) need to be investigated in the aspect of networks. This study aimed to examine how DBS of the bilateral subthalamic nucleus (STN) affects the motor networks of patients with idiopathic PD during motor performance and to show the feasibility of the network analysis using cross-sectional positron emission tomography (PET) images in DBS studies.
MATERIALS AND METHODS
We obtained [15O]H2O PET images from ten patients with PD during a sequential finger-to-thumb opposition task and during the resting state, with DBS-On and DBS-Off at STN. To identify the alteration of motor networks in PD and their changes due to STN-DBS, we applied independent component analysis (ICA) to all the cross-sectional PET images. We analysed the strength of each component according to DBS effects, task effects and interaction effects.
RESULTS
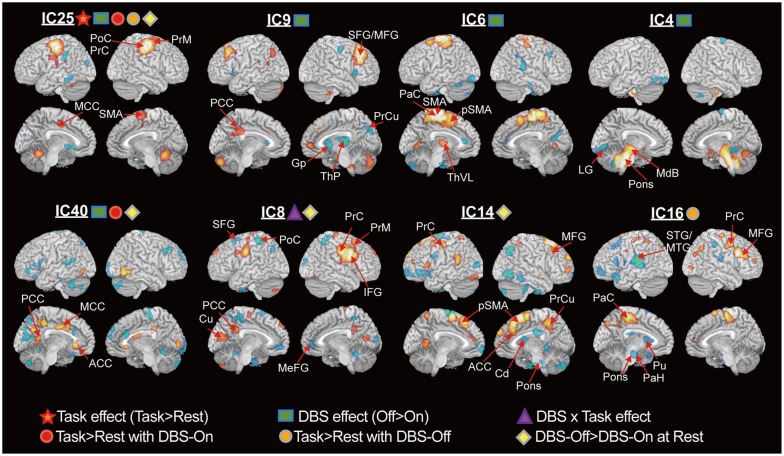
ICA blindly decomposed components of functionally associated distributed clusters, which were comparable to the results of univariate statistical parametric mapping. ICA further revealed that STN-DBS modifies usage-strengths of components corresponding to the basal ganglia-thalamo-cortical circuits in PD patients by increasing the hypoactive basal ganglia and by suppressing the hyperactive cortical motor areas, ventrolateral thalamus and cerebellum.
CONCLUSION
Our results suggest that STN-DBS may affect not only the abnormal local activity, but also alter brain networks in patients with PD. This study also demonstrated the usefulness of ICA for cross-sectional PET data to reveal network modifications due to DBS, which was not observable using the subtraction method.
Keyword
MeSH Terms
Figure
Reference
-
1. Turner RS, Grafton ST, McIntosh AR, DeLong MR, Hoffman JM. The functional anatomy of parkinsonian bradykinesia. Neuroimage. 2003; 19:163–179. PMID: 12781736.
Article2. Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, et al. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain. 1997; 120(Pt 6):963–976. PMID: 9217681.
Article3. Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, et al. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997; 120(Pt 1):103–110. PMID: 9055801.
Article4. Grafton ST, Turner RS, Desmurget M, Bakay R, Delong M, Vitek J, et al. Normalizing motor-related brain activity: subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006; 66:1192–1199. PMID: 16636237.
Article5. Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, et al. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. Neuroimage. 2011; 55:1728–1738. PMID: 21255661.
Article6. Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson's disease. Neuroimage. 2010; 49:2581–2587. PMID: 19853664.
Article7. Clark CM, Kessler R, Buchsbaum MS, Margolin RA, Holcomb HH. Correlational methods for determining regional coupling of cerebral glucose metabolism: a pilot study. Biol Psychiatry. 1984; 19:663–678. PMID: 6610442.8. Horwitz B, Duara R, Rapoport SI. Age differences in intercorrelations between regional cerebral metabolic rates for glucose. Ann Neurol. 1986; 19:60–67. PMID: 3484930.
Article9. Metter EJ, Riege WH, Kuhl DE, Phelps ME. Cerebral metabolic relationships for selected brain regions in healthy adults. J Cereb Blood Flow Metab. 1984; 4:1–7. PMID: 6607258.
Article10. Moeller JR, Strother SC, Sidtis JJ, Rottenberg DA. Scaled subprofile model: a statistical approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab. 1987; 7:649–658. PMID: 3498733.
Article11. Moeller JR, Strother SC. A regional covariance approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab. 1991; 11:A121–A135. PMID: 1997480.
Article12. Park HJ, Kim JJ, Youn T, Lee DS, Lee MC, Kwon JS. Independent component model for cognitive functions of multiple subjects using [15O]H2O PET images. Hum Brain Mapp. 2003; 18:284–295. PMID: 12632466.
Article13. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005; 360:1001–1013. PMID: 16087444.
Article14. McKeown MJ, Sejnowski TJ. Independent component analysis of fMRI data: examining the assumptions. Hum Brain Mapp. 1998; 6:368–372. PMID: 9788074.
Article15. Biswal BB, Ulmer JL. Blind source separation of multiple signal sources of fMRI data sets using independent component analysis. J Comput Assist Tomogr. 1999; 23:265–271. PMID: 10096335.
Article16. Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995; 2:189–210.17. Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995; 3:287–301.
Article18. Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004; 22:1214–1222. PMID: 15219593.
Article19. Hyvärinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999; 10:62634.20. Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, et al. Network modulation in the treatment of Parkinson's disease. Brain. 2006; 129(Pt 10):2667–2678. PMID: 16844713.
Article21. Lozza C, Baron JC, Eidelberg D, Mentis MJ, Carbon M, Marié RM. Executive processes in Parkinson's disease: FDG-PET and network analysis. Hum Brain Mapp. 2004; 22:236–245. PMID: 15195290.
Article22. Moeller JR, Nakamura T, Mentis MJ, Dhawan V, Spetsieres P, Antonini A, et al. Reproducibility of regional metabolic covariance patterns: comparison of four populations. J Nucl Med. 1999; 40:1264–1269. PMID: 10450676.23. Metter EJ, Riege WH, Kameyama M, Kuhl DE, Phelps ME. Cerebral metabolic relationships for selected brain regions in Alzheimer's, Huntington's, and Parkinson's diseases. J Cereb Blood Flow Metab. 1984; 4:500–506. PMID: 6238975.
Article24. Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006; 29:229–257. PMID: 16776585.
Article25. Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003; 61:81621.
Article26. Cilia R, Marotta G, Landi A, Isaias IU, Mariani CB, Vergani F, et al. Clinical and cerebral activity changes induced by subthalamic nucleus stimulation in advanced Parkinson's disease: a prospective case-control study. Clin Neurol Neurosurg. 2009; 111:140–146. PMID: 18995954.
Article27. Geday J, Østergaard K, Johnsen E, Gjedde A. STN-stimulation in Parkinson's disease restores striatal inhibition of thalamocortical projection. Hum Brain Mapp. 2009; 30:112–121. PMID: 18041743.
Article28. Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson's disease. J Cereb Blood Flow Metab. 2004; 24:7–16. PMID: 14688612.
Article29. Karimi M, Golchin N, Tabbal SD, Hershey T, Videen TO, Wu J, et al. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain. 2008; 131(Pt 10):2710–2719. PMID: 18697909.
Article30. Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990; 13:266–271. PMID: 1695401.
Article31. Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989; 12:366–375. PMID: 2479133.
Article32. DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990; 13:281–285. PMID: 1695404.
Article33. Roland PE, Friberg L. The effect of the GABA-A agonist THIP on regional cortical blood flow in humans. A new test of hemispheric dominance. J Cereb Blood Flow Metab. 1988; 8:314–323. PMID: 3366794.
Article34. Takano B, Drzezga A, Peller M, Sax I, Schwaiger M, Lee L, et al. Short-term modulation of regional excitability and blood flow in human motor cortex following rapid-rate transcranial magnetic stimulation. Neuroimage. 2004; 23:849–859. PMID: 15528085.
Article35. Xi ZX, Wu G, Stein EA, Li SJ. GABAergic mechanisms of heroin-induced brain activation assessed with functional MRI. Magn Reson Med. 2002; 48:838–843. PMID: 12417998.
Article36. Chen Z, Silva AC, Yang J, Shen J. Elevated endogenous GABA level correlates with decreased fMRI signals in the rat brain during acute inhibition of GABA transaminase. J Neurosci Res. 2005; 79:383–391. PMID: 15619231.
Article37. Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. 2007; 35:222–233. PMID: 17223579.
Article38. Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb Cortex. 2010; 20:1175–1186. PMID: 19710357.
Article39. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986; 9:357–381. PMID: 3085570.
Article40. Xiong J, Parsons LM, Gao JH, Fox PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp. 1999; 8:151–156. PMID: 10524607.
Article41. Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009; 106:13040–13045. PMID: 19620724.
Article42. Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp. 2009; 30:711–724. PMID: 18266214.
Article43. Di X, Biswal BB. Alzheimer's Disease Neuroimaging Initiative. Metabolic brain covariant networks as revealed by FDG-PET with reference to resting-state fMRI networks. Brain Connect. 2012; 2:275–283. PMID: 23025619.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Striatal Network Model in Parkinson Disease: Preliminary Study
- Update on Current Technologies for Deep Brain Stimulation in Parkinson’s Disease
- Inadequate Efficacy of Deep Brain Stimulation in a Patient with Parkinson's disease due to Partial Breakage of Electrode Lead
- Pallidal Deep Brain Stimulation for Refractory Celiac-Related Myoclonus
- Modulating Neural Network through rTMS