Korean J Ophthalmol.
2019 Apr;33(2):142-149. 10.3341/kjo.2018.0070.
Epiretinal Proliferation Associated with Lamellar Hole or Macular Hole: Origin and Surgical Prognosis
- Affiliations
-
- 1Department of Ophthalmology, HanGil Eye Hospital, Incheon, Korea. jhsohn19@hanafos.com
- 2Department of Pathology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Department of Ophthalmology, Asan Medical Center, Seoul, Korea.
- KMID: 2442619
- DOI: http://doi.org/10.3341/kjo.2018.0070
Abstract
- PURPOSE
To determine the origin of epiretinal proliferation (EP), a condition that is occasionally observed in lamellar hole and macular hole cases, and EP outcomes after vitrectomy.
METHODS
This is a retrospective observational case review of 17 eyes with EP that underwent vitrectomy, EP dissection, and internal limiting membrane peeling between January 2013 and December 2016. Surgical specimens of EP tissue were successfully obtained from 5 cases and they were analyzed after immunohistochemical staining. Postoperative outcomes, including best-corrected visual acuity (BCVA) and macular configuration in spectral domain-optical coherence tomography, were reviewed.
RESULTS
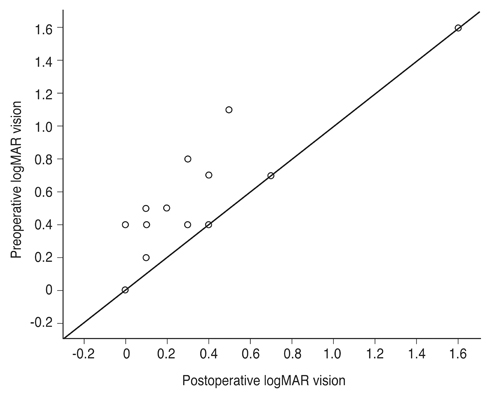
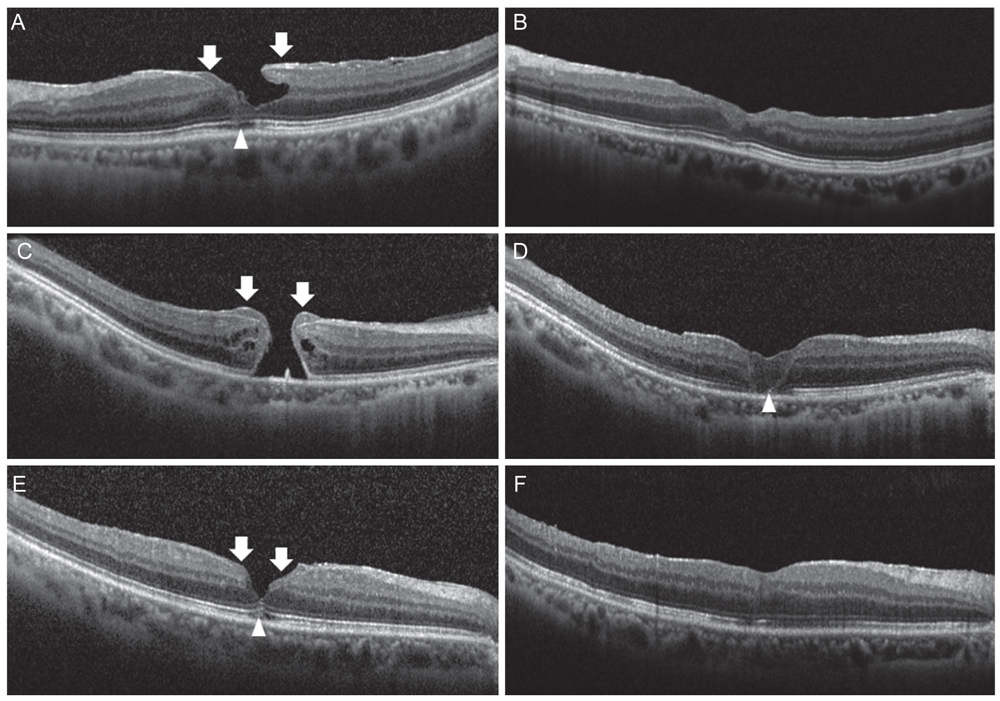
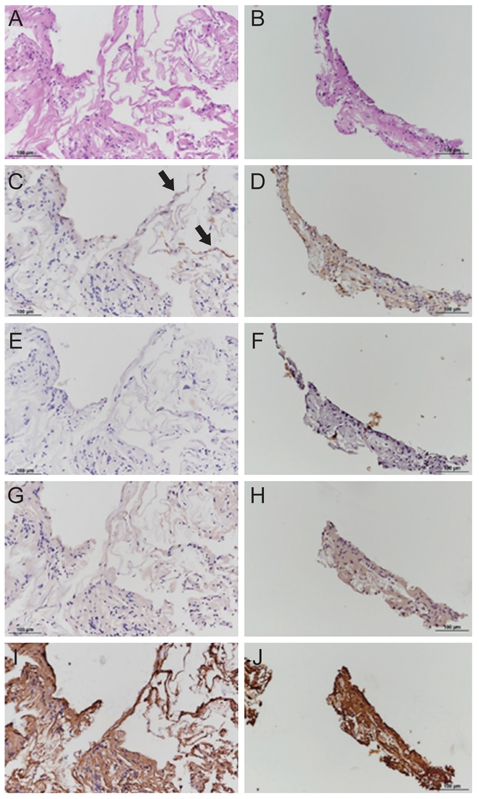
Mean BCVA improved from 0.54 ± 0.36 logarithms of the minimum angle of resolution preoperatively to 0.32 ± 0.38 logarithms of the minimum angle of resolution postoperatively (p = 0.002). BCVA improved in 13 eyes and remained unchanged in four eyes. No cases experienced vision decline after surgery. All 17 patients' lamellar hole or macular hole were successfully closed. Despite hole closure, ellipsoid zone defects were not corrected in 11 of the 17 patients. In immunohistochemical analyses, anti-glial fibrillary acidic protein and pan-keratin (AE1/AE3) were positive, but synaptophysin, anti-α-smooth muscle actin, and anti-CD68 were negative.
CONCLUSIONS
The epiretinal proliferative membrane seems to originate from Müller cells, not from the vitreous. It is unclear whether retinal pigment epithelia also contribute to EP formation. Gentle handling and preservation of the epiretinal proliferative tissue is crucial for successful surgical outcomes.
MeSH Terms
Figure
Reference
-
1. Haouchine B, Massin P, Tadayoni R, et al. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am J Ophthalmol. 2004; 138:732–739.
Article2. Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006; 113:388–397.
Article3. Parolini B, Schumann RG, Cereda MG, et al. Lamellar macular hole: a clinicopathologic correlation of surgically excised epiretinal membranes. Invest Ophthalmol Vis Sci. 2011; 52:9074–9083.
Article4. Michalewska Z, Michalewski J, Odrobina D, Nawrocki J. Non-full-thickness macular holes reassessed with spectral domain optical coherence tomography. Retina. 2012; 32:922–929.
Article5. Bottoni F, Deiro AP, Giani A, et al. The natural history of lamellar macular holes: a spectral domain optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2013; 251:467–475.
Article6. Schumann RG, Compera D, Schaumberger MM, et al. Epiretinal membrane characteristics correlate with photoreceptor layer defects in lamellar macular holes and macular pseudoholes. Retina. 2015; 35:727–735.
Article7. Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014; 34:1513–1523.8. Pang CE, Spaide RF, Freund KB. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina. 2015; 35:720–726.
Article9. Compera D, Entchev E, Haritoglou C, et al. Lamellar hole-associated epiretinal proliferation in comparison to epiretinal membranes of macular pseudoholes. Am J Ophthalmol. 2015; 160:373–384.
Article10. Govetto A, Dacquay Y, Farajzadeh M, et al. Lamellar macular hole: two distinct clinical entities? Am J Ophthalmol. 2016; 164:99–109.
Article11. Lai TT, Chen SN, Yang CM. Epiretinal proliferation in lamellar macular holes and full-thickness macular holes: clinical and surgical findings. Graefes Arch Clin Exp Ophthalmol. 2016; 254:629–638.
Article12. Son G, Lee JS, Lee S, Sohn J. Epiretinal proliferation associated with macular hole and intraoperative perifoveal crown phenomenon. Korean J Ophthalmol. 2016; 30:399–409.
Article13. Compera D, Entchev E, Haritoglou C, et al. Correlative microscopy of lamellar hole-associated epiretinal proliferation. J Ophthalmol. 2015; 2015:450212.
Article14. Ko J, Kim GA, Lee SC, et al. Surgical outcomes of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Acta Ophthalmol. 2017; 95:e221–e226.
Article15. Pang CE, Maberley DA, Freund KB, et al. Lamellar hole-associated epiretinal proliferation: a clinicopathologic correlation. Retina. 2016; 36:1408–1412.16. dell'Omo R, Virgili G, Rizzo S, et al. Role of lamellar hole-associated epiretinal proliferation in lamellar macular holes. Am J Ophthalmol. 2017; 175:16–29.17. Shiraga F, Takasu I, Fukuda K, et al. Modified vitreous surgery for symptomatic lamellar macular hole with epiretinal membrane containing macular pigment. Retina. 2013; 33:1263–1269.
Article18. Heidenkummer HP, Kampik A. Morphologic analysis of epiretinal membranes in surgically treated idiopathic macular foramina. Results of light and electron microscopy. Ophthalmologe. 1996; 93:675–679.19. Kampik A, Kenyon KR, Michels RG, et al. Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch Ophthalmol. 1981; 99:1445–1454.20. Schumann RG, Schaumberger MM, Rohleder M, et al. Ultrastructure of the vitreomacular interface in full-thickness idiopathic macular holes: a consecutive analysis of 100 cases. Am J Ophthalmol. 2006; 141:1112–1119.
Article21. Bringmann A, Pannicke T, Grosche J, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006; 25:397–424.22. Bignami A, Dahl D. The radial glia of Muller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979; 28:63–69.23. Eisenfeld AJ, Bunt-Milam AH, Sarthy PV. Muller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984; 25:1321–1328.24. Kriho VK, Yang HY, Moskal JR, Skalli O. Keratin expression in astrocytomas: an immunofluorescent and biochemical reassessment. Virchows Arch. 1997; 431:139–147.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Visual and Surgical Outcomes of Lamellar Macular Hole
- Spontaneous Disappearance of A Traumatic Macular Hole
- Epiretinal Proliferation Associated with Macular Hole and Intraoperative Perifoveal Crown Phenomenon
- The Evaluation of Prognostic Factors after Vitrectomy for Lamellar Macular Hole Using Optical Coherence Tomography
- Prognostic Factors in Idiopathic Macular Hole Surgery