J Breast Cancer.
2018 Mar;21(1):96-101. 10.4048/jbc.2018.21.1.96.
Malignant Melanoma of the Nipple: A Case Report
- Affiliations
-
- 1Department of Surgery II, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan. y-nagata@med.uoeh-u.ac.jp
- 2Department of Dermatology, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
- 3Tsurudome Breast and Coloproctology Clinic, Kitakyushu, Japan.
- 4Department of Pathology and Cell Biology II, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
- KMID: 2441874
- DOI: http://doi.org/10.4048/jbc.2018.21.1.96
Abstract
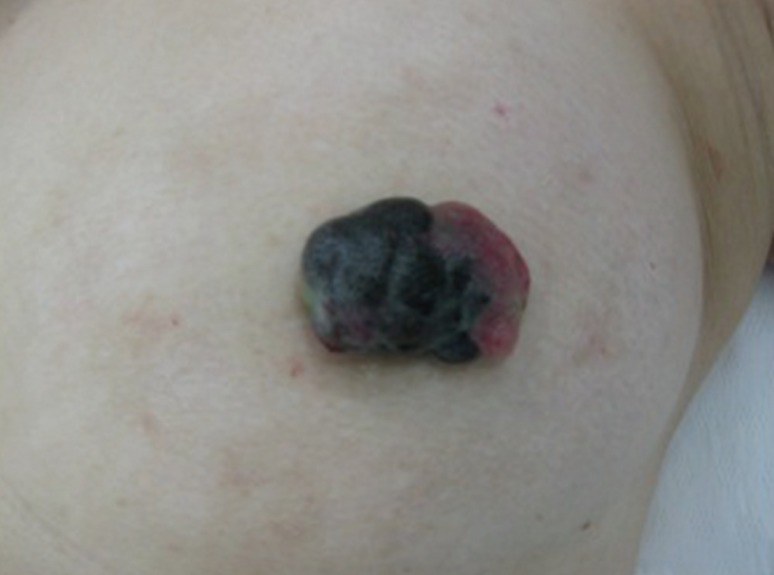
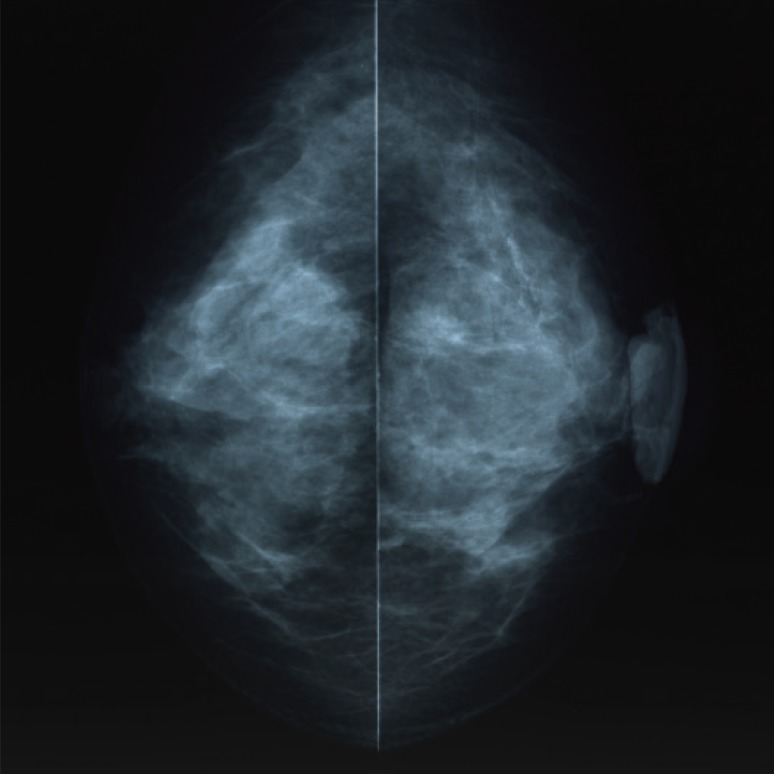
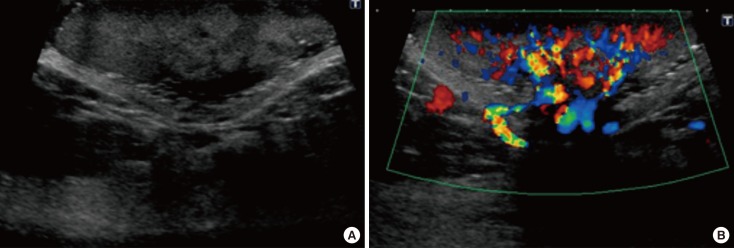
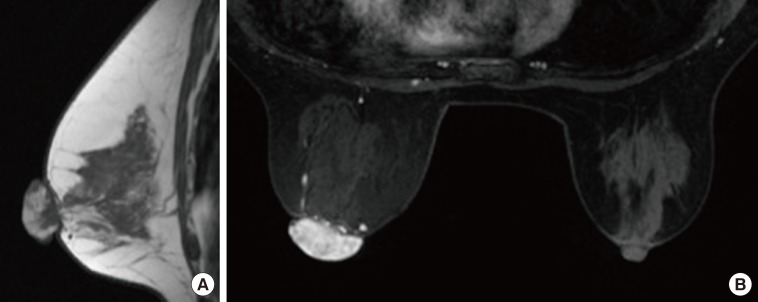
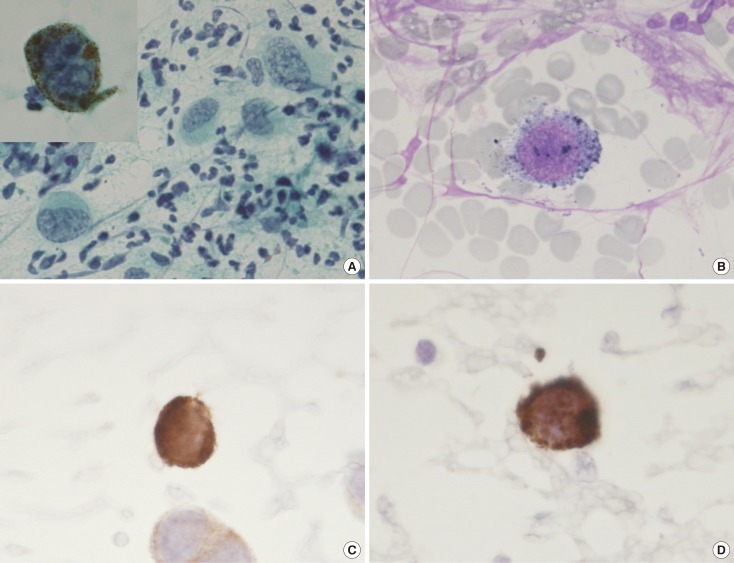
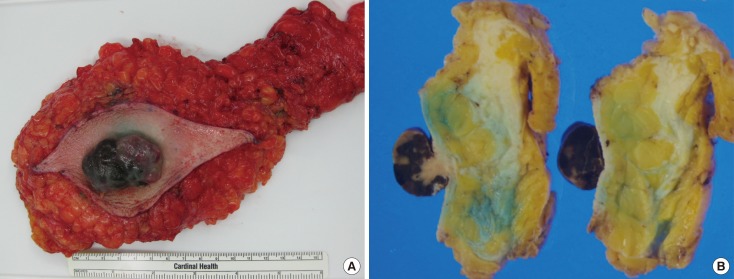
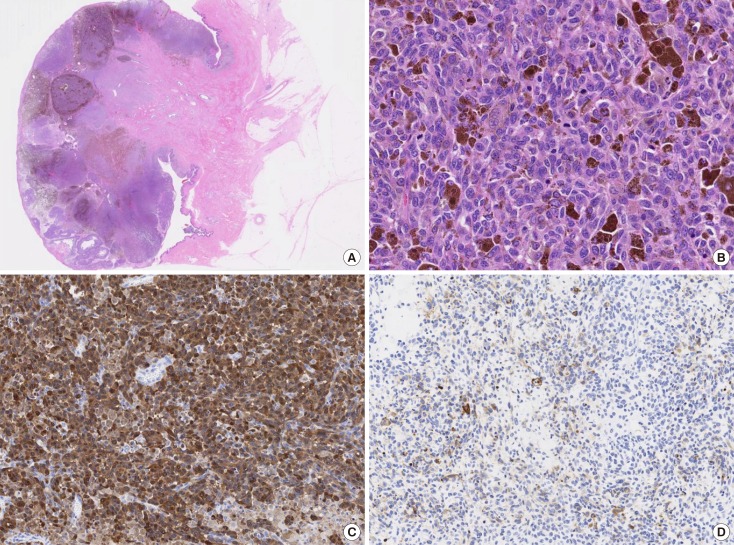
- Malignant melanoma rarely originates from the female nipple. Tumors that develop on the skin of the breast are often subject to a delayed diagnosis. Cytologic examination provides excellent diagnostic capabilities and is a safe procedure with a lower risk of local implantation, compared to needle or incisional biopsy. We herein report a patient who underwent surgical resection of a primary malignant melanoma of the nipple. An elastic soft nodule was observed on the left nipple, and no abnormal lesions were identified in the breast. Eventually, a malignant melanoma was diagnosed from the clinical and cytological evaluation findings. This bulky tumor was classified as a stage IIIC nodular melanoma, with a thickness of 12 mm. The patient received adjuvant chemotherapy and exhibits no evidence of recurrence 7 years after surgery.
Keyword
MeSH Terms
Figure
Reference
-
1. Haagensen CD. Diseases of the Breast. 3rd ed. Philadelphia: WB Saunders;1986. p. 352–356.2. Ariel IM, Caron AS. Diagnosis and treatment of malignant melanoma arising from the skin of the female breast. Am J Surg. 1972; 124:384–390. PMID: 4115719.
Article3. Roses DF, Harris MN, Stern JS, Gumport SL. Cutaneous melanoma of the breast. Ann Surg. 1979; 189:112–115. PMID: 758856.
Article4. Greenberg BM, Hamilton R, Rothkopf DM, Balk M, Clark WH Jr, LaRossa D. Management of cutaneous melanomas of the female breast. Plast Reconstr Surg. 1987; 80:409–415. PMID: 3628570.
Article5. Papachristou DN, Kinne D, Ashikari R, Fortner JG. Melanoma of the nipple and areola. Br J Surg. 1979; 66:287–288. PMID: 455001.
Article6. Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27:6199–6206. PMID: 19917835.
Article7. Ishihara K, Saida T, Otsuka F, Yamazaki N. Prognosis and Statistical Investigation Committee of the Japanese Skin Cancer Society. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008; 13:33–41. PMID: 18307017.
Article8. Mihm MC Jr, Clark WH Jr, From L. The clinical diagnosis, classification and histogenetic concepts of the early stages of cutaneous malignant melanomas. N Engl J Med. 1971; 284:1078–1082. PMID: 4929321.
Article9. Lens MB, Dawes M, Goodacre T, Bishop JA. Excision margins in the treatment of primary cutaneous melanoma: a systematic review of randomized controlled trials comparing narrow vs wide excision. Arch Surg. 2002; 137:1101–1105. PMID: 12361412.
Article10. Haigh PI, DiFronzo LA, McCready DR. Optimal excision margins for primary cutaneous melanoma: a systematic review and meta-analysis. Can J Surg. 2003; 46:419–426. PMID: 14680348.11. Søndergaard K, Schou G. Therapeutic and clinico-pathological factors in the survival of 1,469 patients with primary cutaneous malignant melanoma in clinical stage I: a multivariate regression analysis. Virchows Arch A Pathol Anat Histopathol. 1985; 408:249–258. PMID: 3936264.12. Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006; 355:1307–1317. PMID: 17005948.
Article13. Yamamoto A, Ishihara K. Clinical study of DAV+IFN-beta therapy (combination adjuvant therapy with intravenous DTIC, ACNU and VCR, and local injection of IFN-beta) for malignant melanoma. Int J Immunother. 1996; 12:73–78.