Investig Clin Urol.
2019 Mar;60(2):84-90. 10.4111/icu.2019.60.2.84.
CRTC2 as a novel prognostic biomarker for worse pathologic outcomes and biochemical recurrence after radical prostatectomy in patients with prostate cancer
- Affiliations
-
- 1Department of Urology, Seoul National University Bundang Hospital, Seongnam, Korea. skhong@snubh.org
- 2Department of Urology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2438818
- DOI: http://doi.org/10.4111/icu.2019.60.2.84
Abstract
- PURPOSE
To identify the association between tumor metabolism and prostate cancer (PCa), we investigated the relationship between expression of metabolism-related genes and clinicopathologic outcomes in patients with localized PCa.
MATERIALS AND METHODS
We prospectively collected periprostatic adipose tissue from 40 PCa patients and extracted the RNA of each sample. After cDNA was synthesized from the extracted RNA, we analyzed the expression of 18 metabolism-related genes using real-time polymerase chain reaction. We divided the subjects according to the pathologic Gleason score (pGS) and compared the expression of each gene. Subsequently, the clinicopathologic outcomes were also compared according to the expression of each gene.
RESULTS
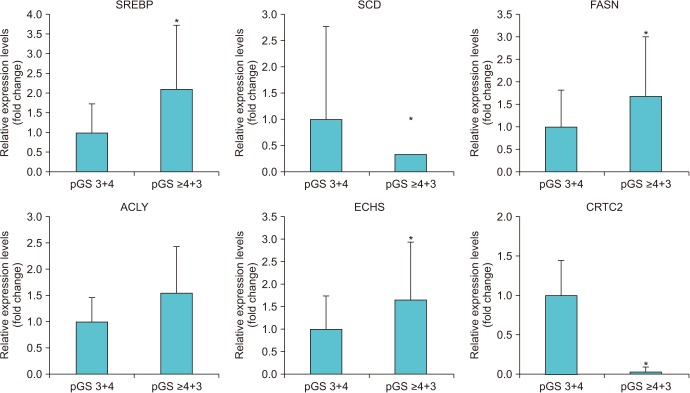
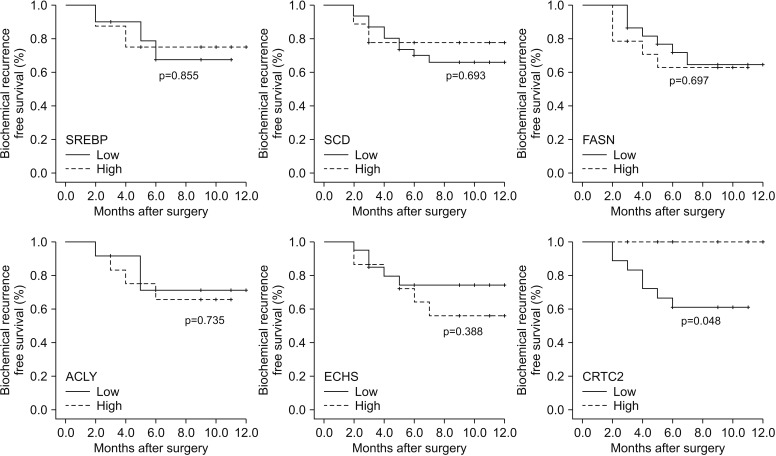
When we compared the expression of 18 metabolism-related genes between the high (≥4+3) and low pGS groups (3+4), there were significant differences in the expression of six genes (SREBP, SCD, FASN, ACLY, ECHS, and CRTC2; p < 0.05). Among them, the subjects with low expression for CRTC2 showed significantly worse pathologic outcomes in terms of high pGS (≥4+3) (p=0.020) and higher rates of seminal vesicle invasion (p=0.017). The low CRTC2 group also showed significantly inferior biochemical recurrence-free survival than the high CRTC2 group (p=0.048).
CONCLUSIONS
We found that high pGS patients showed significant differences in expression of several metabolism-related genes compared with low pGS patients. Among those genes, CRTC2 showed the strongest association with pathologic outcome, as well as postoperative survival.
Keyword
MeSH Terms
Figure
Reference
-
1. Ghagane SC, Nerli RB, Hiremath MB, Wagh AT, Magdum PV. Incidence of prostate cancer at a single tertiary care center in North Karnataka. Indian J Cancer. 2016; 53:429–431. PMID: 28244476.2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29. PMID: 24399786.
Article3. Snowdon DA, Phillips RL, Choi W. Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol. 1984; 120:244–250. PMID: 6465122.4. Freedland SJ, Aronson WJ, Kane CJ, Presti JC Jr, Amling CL, Elashoff D, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004; 22:446–453. PMID: 14691122.
Article5. MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006; 17:989–1003. PMID: 16933050.
Article6. Bergström A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001; 91:421–430. PMID: 11169969.
Article7. Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2011; 4:486–501. PMID: 21233290.
Article8. Freedland SJ, Grubb KA, Yiu SK, Nielsen ME, Mangold LA, Isaacs WB, et al. Obesity and capsular incision at the time of open retropubic radical prostatectomy. J Urol. 2005; 174:1798–1801. PMID: 16217290.
Article9. Wang LS, Murphy CT, Ruth K, Zaorsky NG, Smaldone MC, Sobczak ML, et al. Impact of obesity on outcomes after definitive dose-escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2015; 121:3010–3017. PMID: 26033633.
Article10. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013; 63:800–809. PMID: 23219374.
Article11. Cox ME, Gleave ME, Zakikhani M, Bell RH, Piura E, Vickers E, et al. Insulin receptor expression by human prostate cancers. Prostate. 2009; 69:33–40. PMID: 18785179.
Article12. Lee H, Kuk H, Byun SS, Lee SE, Hong SK. Preoperative glycemic control status as a significant predictor of biochemical recurrence in prostate cancer patients after radical prostatectomy. PLoS One. 2015; 10:e0124761. PMID: 25897669.
Article13. Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004; 363:1346–1353. PMID: 15110491.
Article14. Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer. 2009; 124:2416–2429. PMID: 19142965.
Article15. Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR). Cancer Res. 2001; 61:6276–6280. PMID: 11507082.16. Schnoeller T, Jentzmik F, Rinnab L, Cronauer MV, Damjanoski I, Zengerling F, et al. Circulating free testosterone is an independent predictor of advanced disease in patients with clinically localized prostate cancer. World J Urol. 2013; 31:253–259. PMID: 22763628.
Article17. Lee SE, Chung JS, Han BK, Park CS, Moon KH, Byun SS, et al. Preoperative serum sex hormone-binding globulin as a predictive marker for extraprostatic extension of tumor in patients with clinically localized prostate cancer. Eur Urol. 2008; 54:1324–1332. PMID: 18339472.
Article18. Endogenous Hormones, Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008; 100:170–183. PMID: 18230794.19. Han HS, Choi BH, Kim JS, Kang G, Koo SH. Hepatic Crtc2 controls whole body energy metabolism via a miR-34a-Fgf21 axis. Nat Commun. 2017; 8:1878. PMID: 29192248.
Article20. Wang Y, Inoue H, Ravnskjaer K, Viste K, Miller N, Liu Y, et al. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci U S A. 2010; 107:3087–3092. PMID: 20133702.
Article21. Fang M, Pak ML, Chamberlain L, Xing W, Yu H, Green MR. The CREB coactivator CRTC2 is a lymphoma tumor suppressor that preserves genome integrity through transcription of DNA mismatch repair genes. Cell Rep. 2015; 11:1350–1357. PMID: 26004186.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Prostate Size on Pathologic Outcomes and Prognosis after Radical Prostatectomy
- Radical Prostatectomy
- Biochemical Recurrence-Free and Cancer-Specific Survival after Radical Prostatectomy at a Single Institution
- Role of Radical Prostatectomy for High-Risk Prostate Cancer
- A Case of No Residual Cancer in Radical Prostatectomy Specimens Despite Biopsy-proven Prostate Cancer