Yonsei Med J.
2005 Dec;46(6):818-826.
Differential Expression, Shedding, Cytokine Regulation and Function of TNFR1 and TNFR2 in Human Fetal Astrocytes
- Affiliations
-
- 1Department of Microbiology, Institute of Basic Medical Science, Yonsei University Wonju College of Medicine, Wonju, Korea.
Abstract
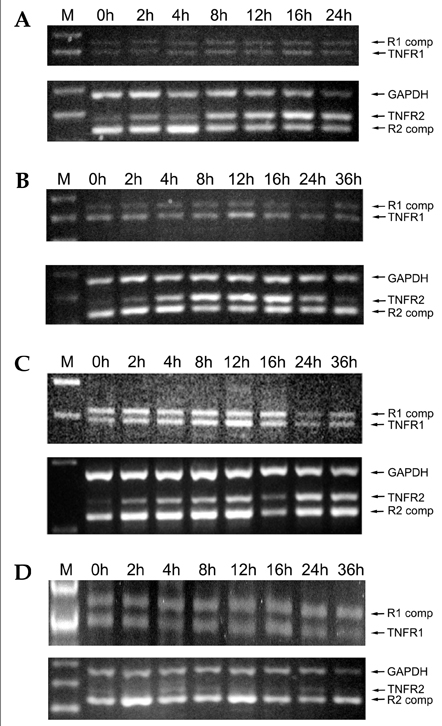
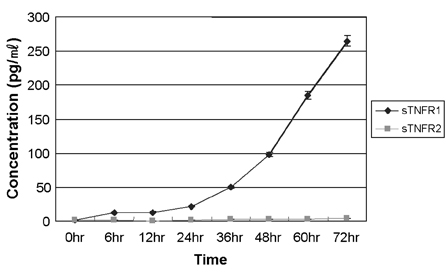
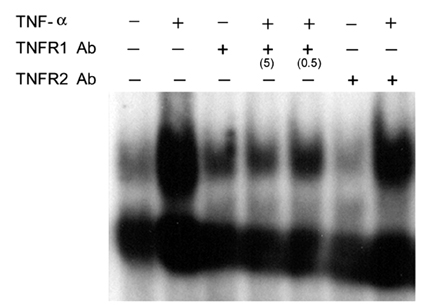
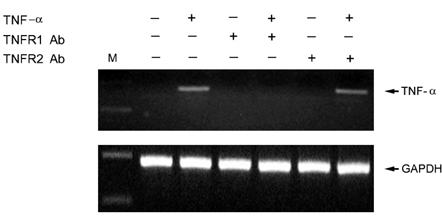
- Tumor necrosis factor (TNF) -alpha induces pleiotropic cellular effects through a 55kDa, type 1 receptor (TNFR1) and a 75kDa type 2 receptor (TNFR2). Moreover, it participates in the pathogenesis of several CNS diseases, including demyelinating diseases. TNF- receptors are differentially expressed and are regulated in many cell types. However, data regarding the TNF-alpha receptor expression and regulation in human astrocytes is limited to date. We investigated TNF-alpha receptor expression, its regulation by cytokines, and its functional role in primary cultured human fetal astrocytes, which are the most abundant cellular population in the central nervous system and are known to be immunologically active. In this study, astrocytes were found to constitutively and predominantly transcribe, translate and shed TNFR1 rather than TNFR2, but TNFR2 expression was increased by adding TNF-alpha, IL-1, and IFN-gamma, but not by adding LPS. To determine the functional roles of TNFR1 and TNFR2 on TNF induction, we investigated NF-kappaB activation and TNF-alpha induction after neutralizing TNFR1 and TNFR2 by an antibody treatment. We found that NF-kappaB activation and TNF-alpha induction are blocked by TNFR1 neutralizing antibody treatments.
Keyword
MeSH Terms
-
Receptors, Tumor Necrosis Factor, Type II/genetics/*metabolism/physiology
Receptors, Tumor Necrosis Factor, Type I/genetics/*metabolism/physiology
RNA, Messenger/metabolism
NF-kappa B/metabolism
Humans
Gene Expression Regulation
Fetus/cytology
Cytokines/*pharmacology
Cells, Cultured
Astrocytes/drug effects/*metabolism
Figure
Reference
-
1. Lee SJ, Drabik K, Van Wagoner NJ, Lee S, Choi C, Dong Y, et al. ICAM-1-induced expression of proinflammatory cytokines in astrocytes: involvement of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. J Immunol. 2000. 165:4658–4666.2. Oh JW, Schwiebert LM, Benveniste EN. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J Neurovirol. 1999. 5:82–94.3. Van Wagoner NJ, Oh JW, Repovic P, Benveniste EN. Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. J Neurosci. 1999. 19:5236–5244.4. Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992. 263(1 pt 1):C1–C16.5. Raine CS. Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest. 1984. 50:608–635.6. Stalder AK, Pagenstecher A, Yu NC, Kincaid C, Chiang CS, Hobbs MV, et al. Lipopolysaccharide-induced IL-12 expression in the central nervous system and cultured astrocytes and microglia. J Immunol. 1997. 159:1344–1351.7. Ledeboer A, Breve JJ, Poole S, Tilders FJ, Van Dam AM. Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. 2000. 30:134–142.8. Ledeboer A, Breve JJ, Wierinckx A, van der Jagt S, Bristow AF, Leysen JE, et al. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur J Neurosci. 2002. 16:1175–1185.9. Martino G, Furlan R, Poliani PL. The pathogenic role of inflammation in multiple sclerosis. Rev Neurol. 2000. 30:1213–1217.10. Benveniste EN, Benos DJ. TNF-alpha- and IFN-gamma-mediated signal transduction pathways: effects on glial cell gene expression and function. FASEB J. 1995. 9:1577–1584.11. Raine CS. Multiple sclerosis: TNF revisited, with promise. Nat Med. 1995. 1:211–214.12. Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992. 13:151–153.13. Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994. 76:959–962.14. Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, et al. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA. 1991. 88:2830–2834.15. Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA. 1991. 88:9292–9296.16. Loetscher H, Stueber D, Banner D, Mackay F, Lesslauer W. Human tumor necrosis factor alpha (TNF alpha) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J Biol Chem. 1993. 268:26350–26357.17. Mackay F, Rothe J, Bluethmann H, Loetscher H, Lesslauer W. Differential responses of fibroblasts from wild-type and TNF-R55-deficient mice to mouse and human TNF-alpha activation. J Immunol. 1994. 153:5274–5284.18. Pandita R, Pocsik E, Aggarwal BB. Interferon-gamma induces cell surface expression for both types of tumor necrosis factor receptors. FEBS Lett. 1992. 312:87–90.19. Winzen R, Wallach D, Kemper O, Resch K, Holtmann H. Selective up-regulation of the 75-kDa tumor necrosis factor (TNF) receptor and its mRNA by TNF and IL-1. J Immunol. 1993. 150:4346–4353.20. Trefzer U, Brockhaus M, Lotscher H, Parlow F, Budnik A, Grewe M, et al. The 55-kD tumor necrosis factor receptor on human keratinocytes is regulated by tumor necrosis factor-alpha and by ultraviolet B radiation. J Clin Invest. 1993. 92:462–470.21. De Kossodo S, Critico B, Grau GE. Modulation of the transcripts for tumor necrosis factor-alpha and its receptors in vivo. Eur J Immunol. 1994. 24:769–772.22. Koller H, Trimborn M, von Giesen H, Schroeter M, Arendt G. TNF alpha reduces glutamate induced intracellular Ca(2+) increase in cultured cortical astrocytes. Brain Res. 2001. 893:237–243.23. Benveniste EN, Sparacio SM, Bethea JR. Tumor necrosis factor-alpha enhances interferon-gamma-mediated class II antigen expression on astrocytes. J Neuroimmunol. 1989. 25:209–219.24. Selmaj KW, Farooq M, Norton WT, Raine CS, Brosnan CF. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990. 144:129–135.25. Munoz-Fernandez MA, Fresno M. Involvement of nitric oxide on the cytokine induced growth of glial cell. Biochem Biophys Res Commun. 1993. 194:319–325.26. Aranguez I, Torres C, Rubio N. The receptor for tumor necrosis factor on murine astrocytes: characterization, intracellular degradation, and regulation by cytokines and Theiler's murine encephalomyelitis virus. Glia. 1995. 13:185–194.27. Lung HL, Leung KN, Stadlin A, Ma CM, Tsang D. Induction of tumor necrosis factor receptor type 2 gene expression by tumor necrosis factor-alpha in rat primary astrocytes. Life Sci. 2001. 68:2081–2091.28. Choi C, Park JY, Lee J, Lim JH, Shin EC, Ahn YS, et al. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999. 162:1889–1895.29. Lee SS, Seo HS, Choi SJ, Park HS, Lee JY, Lee KH, et al. Characterization of the two genes differentially expressed during development in human fetal astrocytes. Yonsei Med J. 2003. 44:1059–1068.30. Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983. 11:1475–1489.31. Chaturvedi MM, LaPushin R, Aggarwal BB. Tumor necrosis factor and lymphotoxin. Qualitative and quantitative differences in the mediation of early and late cellular response. J Biol Chem. 1994. 269:14575–14583.32. Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol. 1997. 75:104–112.33. Tada M, Diserens AC, Desbaillets I, de Tribolet N. Analysis of cytokine receptor messenger RNA expression in human glioblastoma cells and normal astrocytes by reverse-transcription polymerase chain reaction. J Neurosurg. 1994. 80:1063–1073.34. Leeuwenberg JF, Dentener MA, Buurman WA. Lipopolysaccharide LPS-mediated soluble TNF receptor release and TNF receptor expression by monocytes. Role of CD14, LPS binding protein, and bactericidal/permeability-increasing protein. J Immunol. 1994. 152:5070–5076.35. Cope AP, Aderka D, Wallach D, Kahan M, Chu NR, Brennan FM, et al. Soluble TNF receptor production by activated T lymphocytes: differential effects of acute and chronic exposure to TNF. Immunology. 1995. 84:21–30.36. Katsura K, Park M, Gatanaga M, Yu EC, Takishima K, Granger GA, et al. Identification of the proteolytic enzyme which cleaves human p75 TNF receptor in vitro. Biochem Biophys Res Commun. 1996. 222:298–302.37. Kohno T, Brewer MT, Baker SL, Schwartz PE, King MW, Hale KK, et al. A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc Natl Acad Sci USA. 1990. 87:8331–8335.38. Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci USA. 1992. 89:4845–4849.39. Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992. 175:323–329.40. Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, et al. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993. 151:1548–1561.41. Evans TJ, Moyes D, Carpenter A, Martin R, Loetscher H, Lesslauer W, et al. Protective effect of 55- but not 75-kD soluble tumor necrosis factor receptor-immunoglobulin G fusion proteins in an animal model of gram-negative sepsis. J Exp Med. 1994. 180:2173–2179.42. Hale KK, Smith CG, Baker SL, Vanderslice RW, Squires CH, Gleason TM, et al. Multifunctional regulation of the biological effects of TNF-alpha by the soluble type I and type II TNF receptors. Cytokine. 1995. 7:26–38.43. Selmaj K, Shafit-Zagardo B, Aquino DA, Farooq M, Raine CS, Norton WT, et al. Tumor necrosis factor-induced proliferation of astrocytes from mature brain is associated with down-regulation of glial fibrillary acidic protein mRNA. J Neurochem. 1991. 57:823–830.44. Rao P, Hsu KC, Chao MV. Upregulation of NF-kappa B-dependent gene expression mediated by the p75 tumor necrosis factor receptor. J Interferon Cytokine Res. 1995. 15:171–177.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TNFalpha and TNFR2 Immunohistochemistry During Ovarian Follicular Development and Atresia in the Rat
- Role of Clusterin and Tumor Necrosis Factor Receptors on the Apoptosis of Prostate Cancer Cells
- Regulation of TNF - alpha Gene Expression in Human Fetal Astrocytes
- LPS Sensing Mechanism of Human Astrocytes: Evidence of Functional TLR4 Expression and Requirement of Soluble CD14
- Epigenetic Regulation of Cytokine Gene Expression in T Lymphocytes