J Korean Ophthalmol Soc.
2010 May;51(5):728-732.
Effect of Intracameral Triamcinolone to Control Inflammation in Rabbit Eyes
- Affiliations
-
- 1Department of Ophthalmology, Soonchunhyang University College of Medicine, Seoul, Korea. ckseek@hosp.sch.ac.kr
Abstract
- PURPOSE
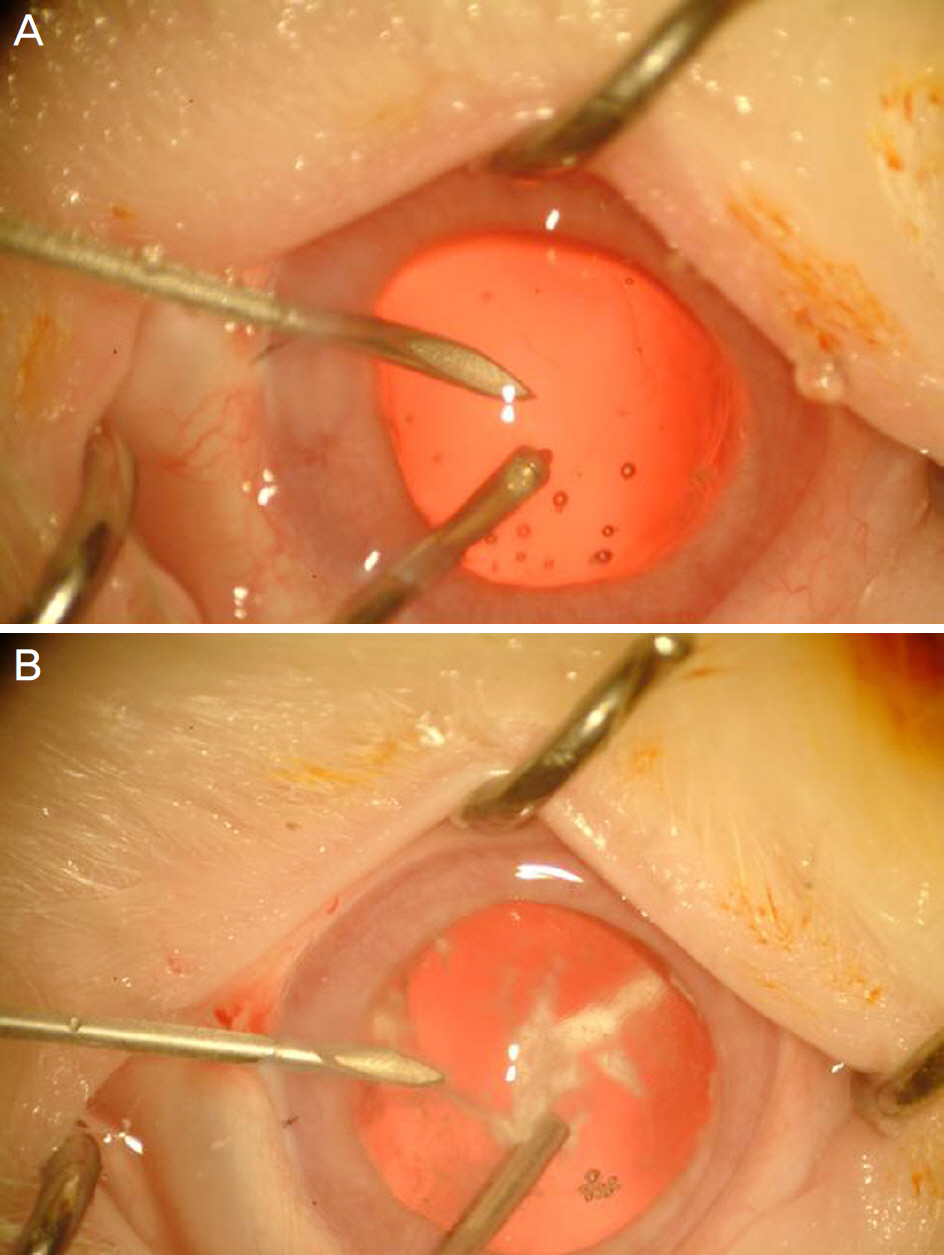
To investigate the effect of intracameral triamcinolone on the control of inflammation with rupture of the posterior lens capsule during cataract surgery in rabbit eyes.
METHODS
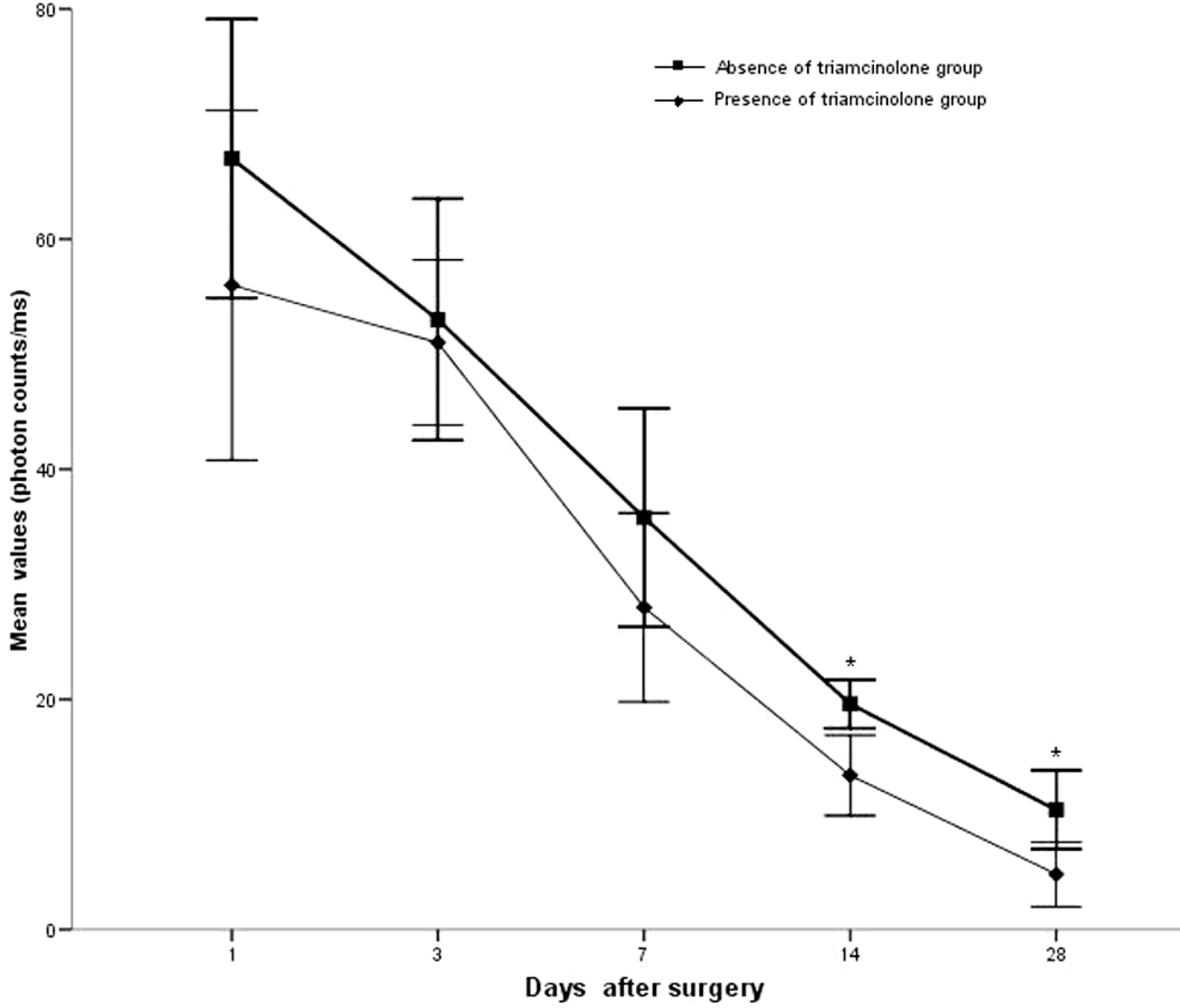
Twenty rabbit eyes were subjected to experimentally induced rupture of the posterior lens capsule and prolapse of the vitreous body into the anterior chamber. After anterior vitrectomy with and without triamcinolone, aqueous flare was measured with a laser flare meter on days 1, 3, 7, 14 and 28.
RESULTS
Vitrectomized eyes with triamcinolone showed a less marked increase in postoperative aqueous flare intensity on days 14 and 28 than did those without triamcinolone (p<0.05).
CONCLUSIONS
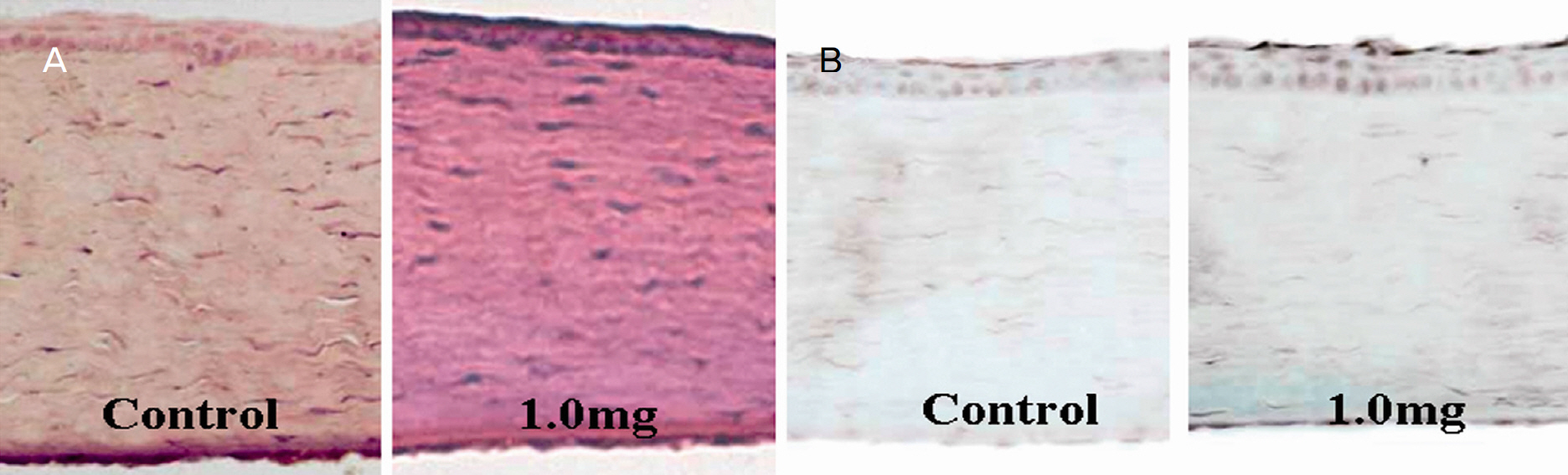
Intracameral injection of triamcinolone was beneficial for visualizing the prolapsed vitreous in the anterior chamber and for helping to control the postoperative inflammation without adverse effects.
MeSH Terms
Figure
Reference
-
References
1. Vajpayee RB, Sharma N, Dada T, et al. Management of posterior capsule tears. Surv Ophthalmol. 2001; 45:473–88.
Article2. Akura J, Hatta S, Kaneda S, et al. Management of posterior abdominal rupture during phacoemulsification using the dry technique. J Cataract Refract Surg. 2001; 27:982–9.3. Yamakiri K, Uchino E, Kimura K, Sakamoto T. Intracameral abdominal helps to visualize and remove the vitreous body in abdominal chamber in cataract surgery. Am J Ophthalmol. 2004; 138:650–2.4. Burk SE, Da Mata AP, Snyder ME, et al. Visualizing vitreous abdominal Kenalog suspension. J Cataract Refract Surg. 2003; 29:645–51.5. Tan DT, Chee SP, Lim L, et al. Randomized clinical trial of Surodex steroid drug delivery system for cataract surgery: abdominal versus posterior placement of two Surodex in the eye. Ophthalmology. 2001; 108:2172–81.6. Karalezli A, Borazan M, Akova YA. Intracameral triamcinolone acetonide to control postoperative inflammation following abdominal surgery with phacoemulsification. Acta Ophthalmol. 2008; 86:183–7.7. Gills JP, Gills P. Effect of intracameral triamcinolone to control inflammation following cataract surgery. J Cataract Refract Surg. 2005; 31:1670–1.
Article8. Walker TD. Benzalkonium toxicity. Clin Experiment Ophthalmol. 2004; 32:657.9. Morrison VL, Koh HJ, Cheng L, et al. Intravitreal toxicity of the kenalog vehicle (benzyl alcohol) in rabbits. Retina. 2006; 26:339–44.
Article10. Garcia-Arumi J, Boixadera A, Giralt J, et al. Comparison of abdominal techniques for purification of triamcinolone acetonide abdominal for intravitreal use. Br J Ophthalmol. 2005; 89:1112–4.11. Nishimura A, Kobayashi A, Segawa Y, et al. Isolating triamcinolone acetonide particles for intravitreal use with a porous abdominal filter. Retina. 2003; 23:777–9.12. Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye. 2005; 19:1133–5.
Article13. Misra A, Burton RL. Incidence of intraoperative complications during phacoemulsification in vitrectomized and nonvitrectomized eyes: prospective study. J Cataract Refract Surg. 2005; 31:1011–4.
Article14. Oh JY, Wee WR, Lee JH, Kim MK. abdominal effect of abdominal triamcinolone acetonide on corneal endothelium using the rabbit model. Eye. 2007; 21:812–8.15. Chang YS, Tseng SY, Tseng SH, et al. Triamcinolone acetonide suspension toxicity to corneal endothelial cells. J Cataract Refract Surg. 2006; 32:1549–55.
Article16. Jonas JB. Intraocular availability of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004; 137:560–2.
Article17. Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single abdominal injection. Ophthalmology. 2003; 110:681–6.18. McCuen BW II, Bessler M, Tano Y, et al. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981; 91:785–8.19. Jonas JB. Concentration of intravitreally injected triamcinolone acetonide in aqueous humour. Br J Ophthalmol. 2002; 86:1066.
Article20. Enaida H, Sakamoto T, Ueno A, et al. Submacular deposition of triamcinolone acetonide after triamcinolone-assisted vitrectomy. Am J Ophthalmol. 2003; 135:243–6.
Article21. O'Brien ET, Perkins SL, Roberts BC, Epstein DL. Dexamethasone inhibits trabecular cell retraction. Exp Eye Res. 1996; 62:675–88.22. Ryu SI, Chang WS, Kim JW, Kim SD. Effects of triamcinolone acetonide in cultured trabecular meshwork cells. J Korean Ophthalmol Soc. 2006; 47:655–60.23. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Intracameral Triamcinolone Acetonide Injection on the Cornea in Rabbits
- Effect of Triamcinolone Acetonide on Proliferative Vitreoretinopathy and Hepatocyte Growth Factor in a Rabbit Model
- Observation on the Effect of Triamcinolone Local Injection in Urethral Syndrome
- Comparison of The Effects of Healon(R) and BioLon(R) on Rabbit Eyeballs after Intracameral and Intravitreal Injections
- Triamcinolone and Glaucoma Filtering Surgery