Yonsei Med J.
2017 May;58(3):479-488. 10.3349/ymj.2017.58.3.479.
Metabolism-Centric Overview of the Pathogenesis of Alzheimer's Disease
- Affiliations
-
- 1Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea. jelee@yuhs
- 2BK21 Plus Project for Medical Sciences and Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2419103
- DOI: http://doi.org/10.3349/ymj.2017.58.3.479
Abstract
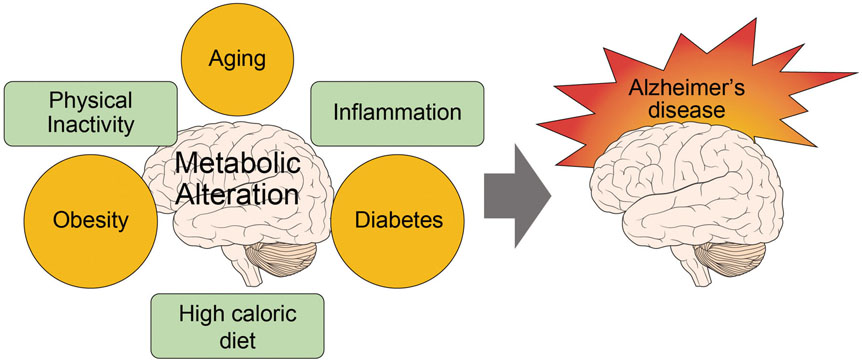
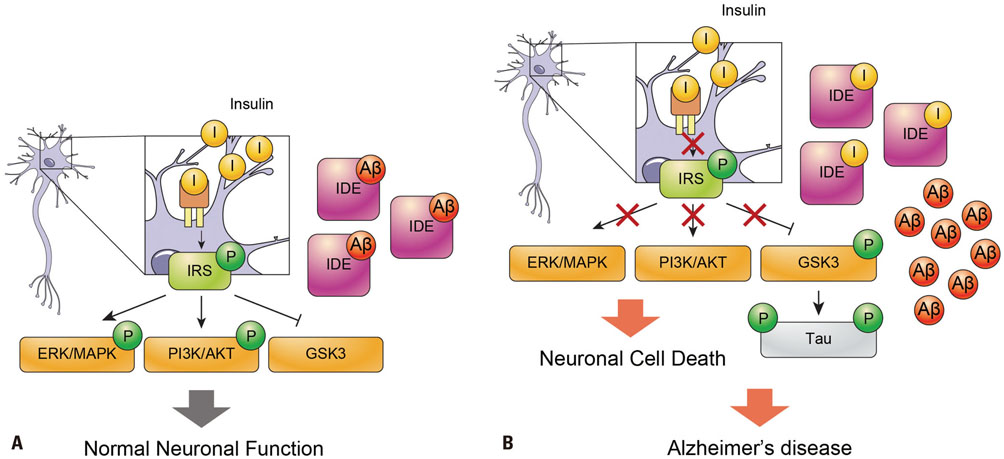
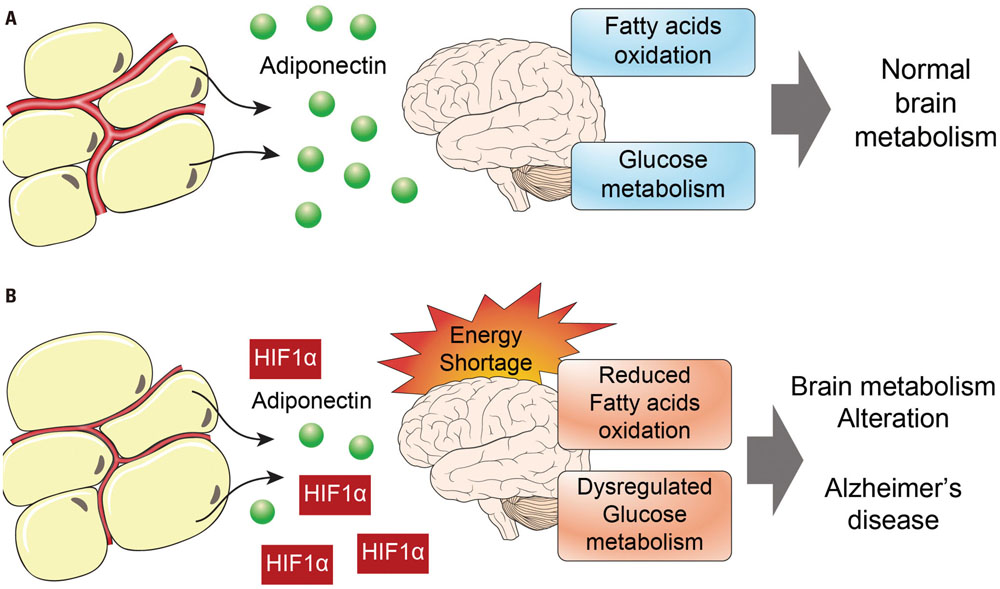
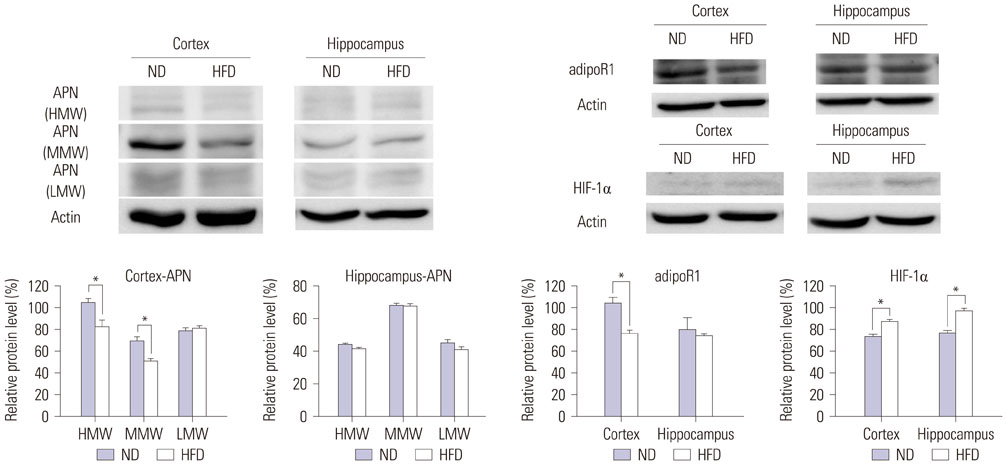
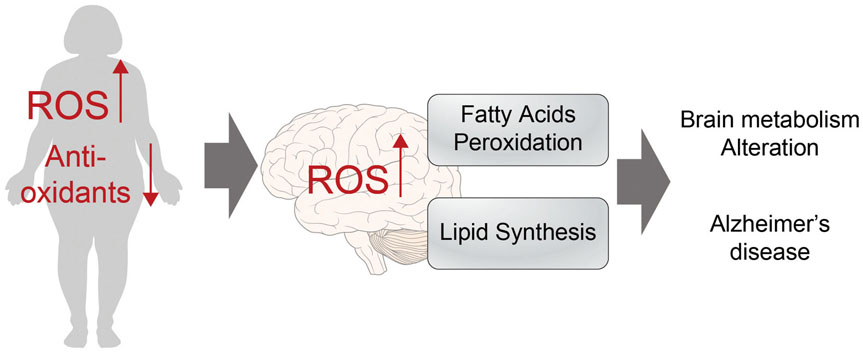
- Alzheimer's disease (AD) is a degenerative brain disease and the most common cause of dementia. AD is characterized by the extracellular amyloid beta (Aβ) plaques and intraneuronal deposits of neurofibrillary tangles (NFTs). Recently, as aging has become a familiar phenomenon around the world, patients with AD are increasing in number. Thus, many researchers are working toward finding effective therapeutics for AD focused on Aβ hypothesis, although there has been no success yet. In this review paper, we suggest that AD is a metabolic disease and that we should focus on metabolites that are affected by metabolic alterations to find effective therapeutics for AD. Aging is associated with not only AD but also obesity and type 2 diabetes (T2DM). AD, obesity, and T2DM share demographic profiles, risk factors, and clinical and biochemical features in common. Considering AD as a kind of metabolic disease, we suggest insulin, adiponectin, and antioxidants as mechanistic links among these diseases and targets for AD therapeutics. Patients with AD show reduced insulin signal transductions in the brain, and intranasal injection of insulin has been found to have an effect on AD treatment. In addition, adiponectin is decreased in the patients with obesity and T2DM. This reduction induces metabolic dysfunction both in the body and the brain, leading to AD pathogenesis. Oxidative stress is known to be induced by Aβ and NFTs, and we suggest that oxidative stress caused by metabolic alterations in the body induce brain metabolic alterations, resulting in AD.
MeSH Terms
-
Adiponectin/blood/metabolism
*Aging/physiology/psychology
Alzheimer Disease/*metabolism/*pathology
Amyloid beta-Peptides
Antioxidants/metabolism
Biomarkers
Brain/*metabolism/pathology/physiopathology
Brain Chemistry/*physiology
Diabetes Mellitus, Type 2/complications/metabolism
Humans
Insulin/blood/metabolism
Obesity/*metabolism
Oxidative Stress/physiology
Adiponectin
Amyloid beta-Peptides
Antioxidants
Biomarkers
Insulin
Figure
Cited by 1 articles
-
Inhibition of miR-128 Abates Aβ-Mediated Cytotoxicity by Targeting PPAR-γ via NF-κB Inactivation in Primary Mouse Cortical Neurons and Neuro2a Cells
Lijiao Geng, Tao Zhang, Wei Liu, Yong Chen
Yonsei Med J. 2018;59(9):1096-1106. doi: 10.3349/ymj.2018.59.9.1096.
Reference
-
1. Alzheimer's Association. 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015; 11:332–384.2. Kim DH. Epidemiology of dementia in Korea. J Korean Med Assoc. 2002; 45:356–360.
Article3. Mucke L. Neuroscience: Alzheimer's disease. Nature. 2009; 461:895–897.4. Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004; 53:474–481.
Article5. Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014; 2:246–255.
Article6. de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008; 2:1101–1113.
Article7. Heydemann A. An overview of murine high fat diet as a model for type 2 diabetes mellitus. J Diabetes Res. 2016; 2016:2902351.
Article8. Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014; 8:375.
Article9. Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: the framingham heart study. Neurobiol Aging. 2005; 26:Suppl 1. 11–16.
Article10. Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015; 9:93–113.
Article11. Moreira PI. Alzheimer's disease and diabetes: an integrative view of the role of mitochondria, oxidative stress, and insulin. J Alzheimers Dis. 2012; 30:Suppl 2. S199–S215.
Article12. Walker JM, Harrison FE. Shared neuropathological characteristics of obesity, type 2 diabetes and Alzheimer's disease: impacts on cognitive decline. Nutrients. 2015; 7:7332–7357.
Article13. Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002; 59:1258–1263.
Article14. Niu L, Han DW, Xu RL, Han B, Zhou X, Wu HW, et al. A high-sugar high-fat diet induced metabolic syndrome shows some symptoms of Alzheimer's disease in rats. J Nutr Health Aging. 2016; 20:509–513.
Article15. Ledreux A, Wang X, Schultzberg M, Granholm AC, Freeman LR. Detrimental effects of a high fat/high cholesterol diet on memory and hippocampal markers in aged rats. Behav Brain Res. 2016; 312:294–304.
Article16. Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, et al. Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer's disease. Mediators Inflamm. 2015; 2015:105828.
Article17. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016; 8:595–608.
Article18. Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014; 6:37.
Article19. Morris GP, Clark IA, Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol Commun. 2014; 2:135.
Article20. Mullane K, Williams M. Alzheimer's therapeutics: continued clinical failures question the validity of the amyloid hypothesisbut what lies beyond. Biochem Pharmacol. 2013; 85:289–305.
Article21. Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med. 2012; 2:a006213.
Article22. Jeong DU, Oh JH, Lee JE, Lee J, Cho ZH, Chang JW, et al. Basal forebrain cholinergic deficits reduce glucose metabolism and function of cholinergic and GABAergic systems in the cingulate cortex. Yonsei Med J. 2016; 57:165–172.
Article23. Lu Y, Ren J, Cui S, Chen J, Huang Y, Tang C, et al. Cerebral glucose metabolism assessment in rat models of Alzheimer's disease: an 18F-FDG-PET study. Am J Alzheimers Dis Other Demen. 2016; 31:333–340.
Article24. Liguori C, Chiaravalloti A, Sancesario G, Stefani A, Sancesario GM, Mercuri NB, et al. Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2016; 43:2040–2049.
Article25. Krell-Roesch J, Ruider H, Lowe VJ, Stokin GB, Pink A, Roberts RO, et al. FDG-PET and neuropsychiatric symptoms among cognitively normal elderly persons: the mayo clinic study of aging. J Alzheimers Dis. 2016; 53:1609–1616.
Article26. Campion D, Pottier C, Nicolas G, Le Guennec K, Rovelet-Lecrux A. Alzheimer disease: modeling an Aβ-centered biological network. Mol Psychiatry. 2016; 21:861–871.
Article27. Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011; 14:724–738.
Article28. Allaman I, Bélanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011; 34:76–87.
Article29. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006; 443:787–795.
Article30. Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007; 12:913–922.
Article31. Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the rotterdam study. Neurology. 1999; 53:1937–1942.
Article32. Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, et al. Glucose levels and risk of dementia. N Engl J Med. 2013; 369:540–548.
Article33. Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer's disease. Exp Gerontol. 2007; 42:10–21.
Article34. Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005; 7:63–80.
Article35. Leng Y, Karlsson HK, Zierath JR. Insulin signaling defects in type 2 diabetes. Rev Endocr Metab Disord. 2004; 5:111–117.
Article36. Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. 2014; 220:T1–T23.37. Roberts LD, Koulman A, Griffin JL. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. Lancet Diabetes Endocrinol. 2014; 2:65–75.
Article38. Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004; 490:5–12.
Article39. Gray SM, Meijer RI, Barrett EJ. Insulin regulates brain function, but how does it get there? Diabetes. 2014; 63:3992–3997.
Article40. Medhi B, Chakrabarty M. Insulin resistance: an emerging link in Alzheimer's disease. Neurol Sci. 2013; 34:1719–1725.
Article41. Goberdhan DC, Wilson C. The functions of insulin signaling: size isn't everything, even in Drosophila. Differentiation. 2003; 71:375–397.
Article42. de la Monte SM, Chen GJ, Rivera E, Wands JR. Neuronal thread protein regulation and interaction with microtubule-associated proteins in SH-Sy5y neuronal cells. Cell Mol Life Sci. 2003; 60:2679–2691.
Article43. Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005; 26:916–943.
Article44. Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005; 16:59–65.
Article45. Kleinridders A, Ferris HA, Cai W, Kahn CR. nsulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014; 63:2232–2243.
Article46. McNay EC. Insulin and ghrelin: peripheral hormones modulating memory and hippocampal function. Curr Opin Pharmacol. 2007; 7:628–632.
Article47. Heni M, Kullmann S, Preissl H, Fritsche A, Häring HU. Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol. 2015; 11:701–711.
Article48. De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer's disease? Alzheimers Dement. 2014; 10:1 Suppl. S26–S32.
Article49. Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008; 22:246–260.
Article50. Ho L, Yemul S, Knable L, Katsel P, Zhao R, Haroutunian V, et al. Insulin receptor expression and activity in the brains of nondiabetic sporadic Alzheimer's disease cases. Int J Alzheimers Dis. 2012; 2012:321280.
Article51. Kang S, Kim CH, Jung H, Kim E, Song HT, Lee JE. Agmatine ameliorates type 2 diabetes induced-Alzheimer's disease-like alterations in high-fat diet-fed mice via reactivation of blunted insulin signalling. Neuropharmacology. 2017; 113(Pt A):467–479.
Article52. Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003; 100:4162–4167.
Article53. Shiiki T, Ohtsuki S, Kurihara A, Naganuma H, Nishimura K, Tachikawa M, et al. Brain insulin impairs amyloid-beta(1-40) clearance from the brain. J Neurosci. 2004; 24:9632–9637.54. Jeon S, Park JE, Lee J, Liu QF, Jeong HJ, Pak SC, et al. Illite improves memory impairment and reduces Aβ level in the Tg-APPswe/PS1dE9 mouse model of Alzheimer's disease through Akt/CREB and GSK-3β phosphorylation in the brain. J Ethnopharmacol. 2015; 160:69–77.
Article55. Farr SA, Sandoval KE, Niehoff ML, Witt KA, Kumar VB, Morley JE, et al. Peripheral administration of GSK-3β antisense oligonucleotide improves learning and memory in SAMP8 and Tg2576 mouse models of Alzheimer's disease. J Alzheimers Dis. 2016; 54:1339–1348.
Article56. Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004; 29:1326–1334.
Article57. Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012; 69:29–38.
Article58. Smith KB, Smith MS. Obesity statistics. Prim Care. 2016; 43:121–135.
Article59. Pataky Z, Bobbioni-Harsch E, Golay A. Obesity: a complex growing challenge. Exp Clin Endocrinol Diabetes. 2010; 118:427–433.
Article60. Emmerzaal TL, Kiliaan AJ, Gustafson DR. 2003-2013: a decade of body mass index, Alzheimer's disease, and dementia. J Alzheimers Dis. 2015; 43:739–755.
Article61. Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014; 220:T47–T59.
Article62. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011; 11:85–97.
Article63. Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci (Lond). 2013; 124:491–507.
Article64. López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, et al. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014; 18:37–45.
Article65. Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001; 7:947–953.
Article66. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007; 117:2621–2637.
Article67. Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. 2014; 15:149–156.
Article68. Maddineni S, Metzger S, Ocón O, Hendricks G 3rd, Ramachandran R. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology. 2005; 146:4250–4256.
Article69. Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012; 165:313–327.
Article70. Letra L, Santana I, Seiça R. Obesity as a risk factor for Alzheimer's disease: the role of adipocytokines. Metab Brain Dis. 2014; 29:563–568.
Article71. Yang Y, Hu W, Jiang S, Wang B, Li Y, Fan C, et al. The emerging role of adiponectin in cerebrovascular and neurodegenerative diseases. Biochim Biophys Acta. 2015; 1852:1887–1894.
Article72. Ramamurthy S, Ronnett GV. Developing a head for energy sensingsensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol. 2006; 574(Pt 1):85–93.
Article73. Girgis CM, Cheng K, Scott CH, Gunton JE. Novel links between HIFs, type 2 diabetes, and metabolic syndrome. Trends Endocrinol Metab. 2012; 23:372–380.
Article74. Jiang C, Kim JH, Li F, Qu A, Gavrilova O, Shah YM, et al. Hypoxiainducible factor 1α regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. J Biol Chem. 2013; 288:3844–3857.
Article75. Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought. Neurochem Int. 2004; 45:321–351.
Article76. Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res. 2001; 66:824–838.
Article77. Dhopeshwarkar GA, Mead JF. Fatty acid uptake by the brain. 3. Incorporation of (1-14C)oleic acid into the adult rat brain. Biochim Biophys Acta. 1970; 210:250–256.78. Panov A, Orynbayeva Z, Vavilin V, Lyakhovich V. Fatty acids in energy metabolism of the central nervous system. Biomed Res Int. 2014; 2014:472459.
Article79. Schönfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab. 2013; 33:1493–1499.
Article80. Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015; 6:456–480.
Article81. Cossarizza A, Ferraresi R, Troiano L, Roat E, Gibellini L, Bertoncelli L, et al. Simultaneous analysis of reactive oxygen species and reduced glutathione content in living cells by polychromatic flow cytometry. Nat Protoc. 2009; 4:1790–1797.
Article82. Navarro-Yepes J, Burns M, Anandhan A, Khalimonchuk O, del Razo LM, Quintanilla-Vega B, et al. Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid Redox Signal. 2014; 21:66–85.
Article83. Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011; 194:7–15.
Article84. Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997; 82:291–295.
Article85. Opazo C, Huang X, Cherny RA, Moir RD, Roher AE, White AR, et al. Metalloenzyme-like activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2). J Biol Chem. 2002; 277:40302–40308.86. Giraldo E, Lloret A, Fuchsberger T, Viña J. Aβ and tau toxicities in Alzheimer's are linked via oxidative stress-induced p38 activation: protective role of vitamin E. Redox Biol. 2014; 2:873–877.
Article87. Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002; 23:655–664.88. Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond). 2006; 30:400–418.
Article89. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–1761.
Article90. Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002; 5:561–568.
Article91. Butterfield DA, Swomley AM, Sultana R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal. 2013; 19:823–835.
Article92. Savaskan NE, Ufer C, Kühn H, Borchert A. Molecular biology of glutathione peroxidase 4: from genomic structure to developmental expression and neural function. Biol Chem. 2007; 388:1007–1017.
Article93. Seghrouchni I, Drai J, Bannier E, Rivière J, Calmard P, Garcia I, et al. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus; insulin treatment efficiency. Clin Chim Acta. 2002; 321:89–96.
Article94. Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, et al. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006; 26:5167–5179.
Article95. Mailloux RJ, Bériault R, Lemire J, Singh R, Chénier DR, Hamel RD, et al. The tricarboxylic acid cycle, an ancient metabolic network with a novel twist. PLoS One. 2007; 2:e690.
Article96. Mailloux RJ, Puiseux-Dao S, Appanna VD. Alpha-ketoglutarate abrogates the nuclear localization of HIF-1alpha in aluminum-exposed hepatocytes. Biochimie. 2009; 91:408–415.
Article97. Bianchetti A, Rozzini R, Trabucchi M. Effects of acetyl-L-carnitine in Alzheimer's disease patients unresponsive to acetylcholinesterase inhibitors. Curr Med Res Opin. 2003; 19:350–353.
Article98. Stark AK, Pelvig DP, Jørgensen AM, Andersen BB, Pakkenberg B. Measuring morphological and cellular changes in Alzheimer's dementia: a review emphasizing stereology. Curr Alzheimer Res. 2005; 2:449–481.
Article99. Bhatti AB, Usman M, Ali F, Satti SA. Vitamin supplementation as an adjuvant treatment for Alzheimer's disease. J Clin Diagn Res. 2016; 10:OE07–OE11.
Article100. Song J, Hur BE, Bokara KK, Yang W, Cho HJ, Park KA, et al. Agmatine improves cognitive dysfunction and prevents cell death in a streptozotocin-induced Alzheimer rat model. Yonsei Med J. 2014; 55:689–699.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Non-Alzheimer Dementia
- A study on the cephalometric changes by the displacement of the mandibular condyles
- Memorials of Alois Alzheimer (June 14, 1864~December 19, 1915) and Historical Background of Alzheimer's Disease
- A clinical study on the centric discrepancy in postorthodontic patients
- Traumatic Brain Injury in the Elderly