Clin Exp Otorhinolaryngol.
2017 Dec;10(4):357-362. 10.21053/ceo.2016.01095.
Age-Related Changes in Nuclear Factor Erythroid 2-Related Factor 2 and Reactive Oxygen Species and Mitochondrial Structure in the Tongues of Fischer 344 Rats
- Affiliations
-
- 1Department of Otolaryngology Head and Neck Surgery, Gachon University Gil Hospital, Incheon, Korea. hndyk@gilhospital.com
- 2Department of Neurology, Gachon University Gil Hospital, Incheon, Korea.
- 3Department of Medical Research Institute, Gachon University Gil Hospital, Incheon, Korea.
- 4Lee Gil Ya Cancer and Diabetes Institute, Gachon University, Incheon, Korea.
- KMID: 2396792
- DOI: http://doi.org/10.21053/ceo.2016.01095
Abstract
OBJECTIVES
Previously the authors reported age-related changes in the activities of anti-oxidative enzyme activities and protein expressions in the tongues of rats. Because more information is required about relations between aging and oxidative stress and anti-oxidative enzyme efficiency, the authors investigated differences between the expression of master regulator of anti-oxidative enzymes (nuclear factor erythroid 2-related factor 2 [Nrf2]), levels of reactive oxygen species (ROS), and mitochondrial structures in the tongues of young and aged Fischer 344 rats.
METHODS
Age-dependent changes in Nrf2 protein and ROS were determined by Western blotting and using chemical kits, respectively. Tongue specimens were examined by electron microscopy. The study was conducted using rats aged 7 months (young, n=8) or 22 months (old, n=8).
RESULTS
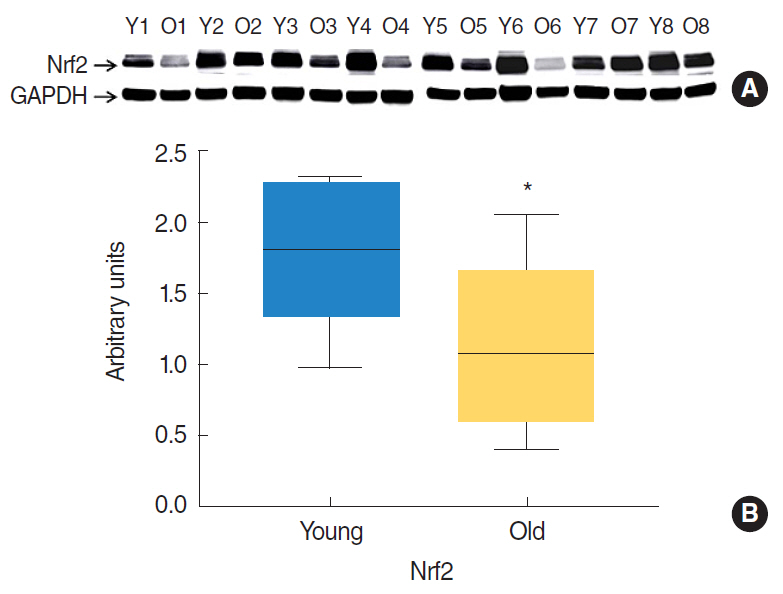
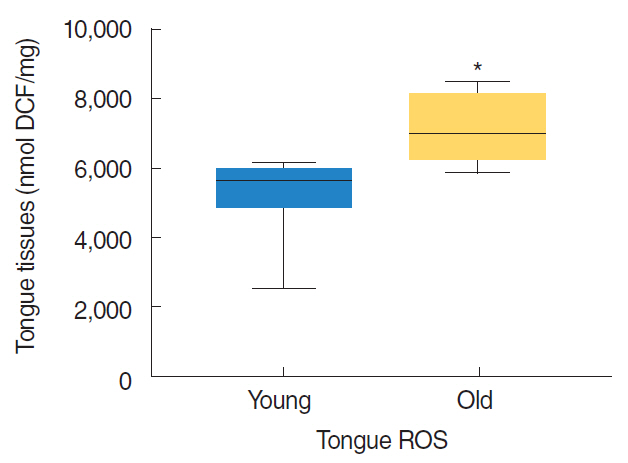
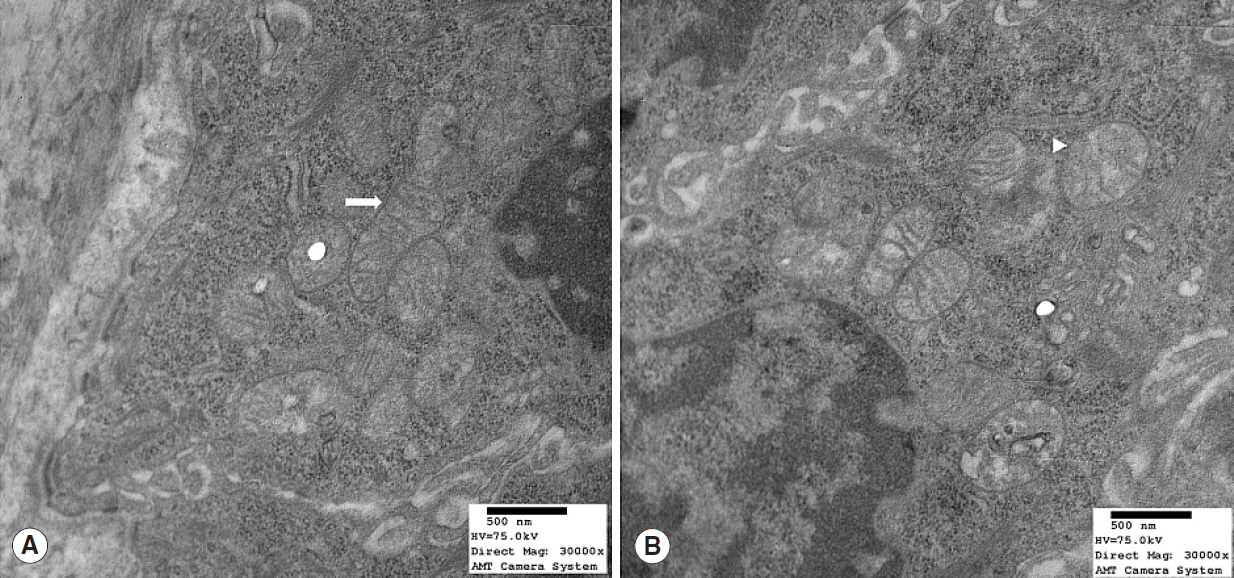
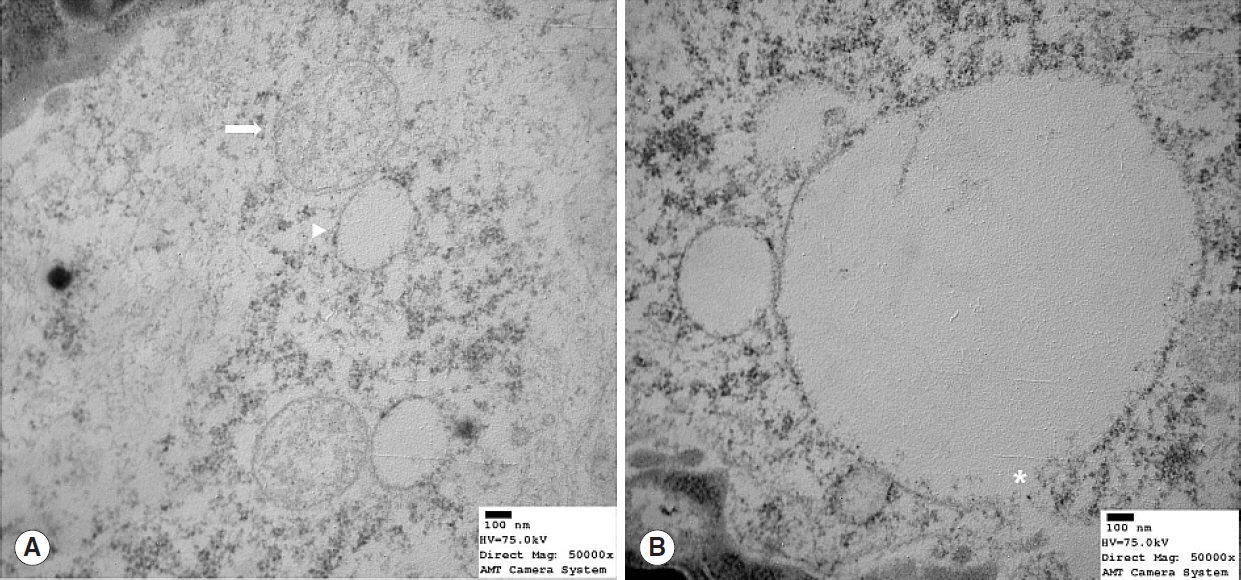
Nrf2 protein levels in the tongues of aged rats were lower than in young rats. ROS levels were higher in older rats and mitochondrial structural deficits were observed their tongues. Three young rats showed moderate mitochondrial degeneration, whereas profound degeneration with mitochondrial cristae disruption, swelling, rupture, or intramitochondrial vacuole formation was observed in all 8 old rats. Notably, mitochondrial rupture was observed in 5 old rats.
CONCLUSION
Antioxidant defense systems of old rats were compromised by Nrf2 deficiency, which could lead to the deleterious accumulation and release of ROS and probably mitochondrial structural deficits in aged tongue tissues.
MeSH Terms
Figure
Reference
-
1. Kirkwood TB. Understanding the odd science of aging. Cell. 2005; Feb. 120(4):437–47.
Article2. Huang JH, Hood DA. Age-associated mitochondrial dysfunction in skeletal muscle: contributing factors and suggestions for long-term interventions. IUBMB Life. 2009; Mar. 61(3):201–14.
Article3. Geokas MC, Conteas CN, Majumdar AP. The aging gastrointestinal tract, liver, and pancreas. Clin Geriatr Med. 1985; Feb. 1(1):177–205.
Article4. Vinokur V, Grinberg L, Berenshtein E, Gross M, Moskovitz J, Reznick AZ, et al. Methionine-centered redox cycle in organs of the aero-digestive tract of young and old rats. Biogerontology. 2009; Feb. 10(1):43–52.5. Baek MK, Kim KO, Kwon HJ, Kim YW, Woo JH, Kim DY. Age-related changes in antioxidative enzyme capacity in tongue of fischer 344 rats. Clin Exp Otorhinolaryngol. 2016; Dec. 9(4):352–7.
Article6. Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011; Feb. 470(7334):359–65.
Article7. Meng Q, Wong YT, Chen J, Ruan R. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer 344 rats. Mech Ageing Dev. 2007; Mar. 128(3):286–92.
Article8. Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003; 27(4):277–84.9. Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004; Nov. 10(11):549–57.
Article10. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997; Jul. 236(2):313–22.
Article11. Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999; Jan. 13(1):76–86.
Article12. Moinova HR, Mulcahy RT. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun. 1999; Aug. 261(3):661–8.13. Ohkoshi A, Suzuki T, Ono M, Kobayashi T, Yamamoto M. Roles of Keap1-Nrf2 system in upper aerodigestive tract carcinogenesis. Cancer Prev Res (Phila). 2013; Feb. 6(2):149–59.
Article14. Smith EJ, Shay KP, Thomas NO, Butler JA, Finlay LF, Hagen TM. Age-related loss of hepatic Nrf2 protein homeostasis: potential role for heightened expression of miR-146a. Free Radic Biol Med. 2015; Dec. 89:1184–91.
Article15. Quinn R. Comparing rat’s to human’s age: how old is my rat in people years. Nutrition. 2005; Jun. 21(6):775–7.
Article16. Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012; 2012:137289.
Article17. Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach. Nat Rev Drug Discov. 2009; Jul. 8(7):579–91.
Article18. Harris IS, Blaser H, Moreno J, Treloar AE, Gorrini C, Sasaki M, et al. PTPN12 promotes resistance to oxidative stress and supports tumorigenesis by regulating FOXO signaling. Oncogene. 2014; Feb. 33(8):1047–54.
Article19. Sohal RS, Ku HH, Agarwal S. Biochemical correlates of longevity in two closely related rodent species. Biochem Biophys Res Commun. 1993; Oct. 196(1):7–11.
Article20. Ceballos-Picot I, Nicole A, Clement M, Bourre JM, Sinet PM. Age-related changes in antioxidant enzymes and lipid peroxidation in brains of control and transgenic mice overexpressing copper-zinc superoxide dismutase. Mutat Res. 1992; 275(3-6):281–93.
Article21. Kumaran S, Savitha S, Anusuya Devi M, Panneerselvam C. L-carnitine and DL-alpha-lipoic acid reverse the age-related deficit in glutathione redox state in skeletal muscle and heart tissues. Mech Ageing Dev. 2004; Jul. 125(7):507–12.22. Beal MF. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses. Ann Neurol. 1992; Feb. 31(2):119–30.
Article23. Diplock AT. Antioxidants and disease prevention. Mol Aspects Med. 1994; 15(4):293–376.
Article24. Terman A, Gustafsson B, Brunk UT. The lysosomal-mitochondrial axis theory of postmitotic aging and cell death. Chem Biol Interact. 2006; Oct. 163(1-2):29–37.
Article25. de Cavanagh EM, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, et al. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J. 2003; Jun. 17(9):1096–8.
Article26. Kumaran S, Panneerselvam KS, Shila S, Sivarajan K, Panneerselvam C. Age-associated deficit of mitochondrial oxidative phosphorylation in skeletal muscle: role of carnitine and lipoic acid. Mol Cell Biochem. 2005; Dec. 280(1-2):83–9.
Article27. Beregi E, Regius O, Huttl T, Gobl Z. Age-related changes in the skeletal muscle cells. Z Gerontol. 1988; Mar-Apr. 21(2):83–6.28. Barlagiannis D, Dietrich E, Papaliagkas V, Makri S, Toskas A, Papamitsou T. Ultrastructural aspects of the effects of L-carnitine administration on epithelial cells in the aging rat tongue. Hippokratia. 2014; Jan. 18(1):32–6.29. Takahashi T. Structual changes of the apex region of the tongue in the elderly. Kokubyo Gakkai Zasshi. 2008; Jun. 75(2):93–105.
Article30. Choi JS, Park IS, Kim SK, Lim JY, Kim YM. Analysis of age-related changes in the functional morphologies of salivary glands in mice. Arch Oral Biol. 2013; Nov. 58(11):1635–42.
Article