Ann Dermatol.
2011 Nov;23(4):439-447.
Retinoid Induces the Degradation of Corneodesmosomes and Downregulation of Corneodesmosomal Cadherins: Implications on the Mechanism of Retinoid-induced Desquamation
- Affiliations
-
- 1Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea. kimsc@yuhs.ac
Abstract
- BACKGROUND
Topical retinoids induce skin fragility. As corneodesmosomes are important adhesion structures in the epidermal cohesion, an effect of retinoids on corneodesmosomes has been suspected.
OBJECTIVE
The aim of this study was to investigate the effect of retinoid on the expression of corneodesmosomal components including desmoglein (DSG) 1, desmocollin (DSC) 1, corneodesmosin (CDSN) and kallikrein (KLK)s.
METHODS
2% all-trans-retinol or ethanol was applied to the back of hairless mice for five days, and the structure of the stratum corneum was examined by transmission electron microscopy. The cultured human keratinocytes were treated with all-trans-retinoic acid (RA) in low or high calcium media for 24 hours.
RESULTS
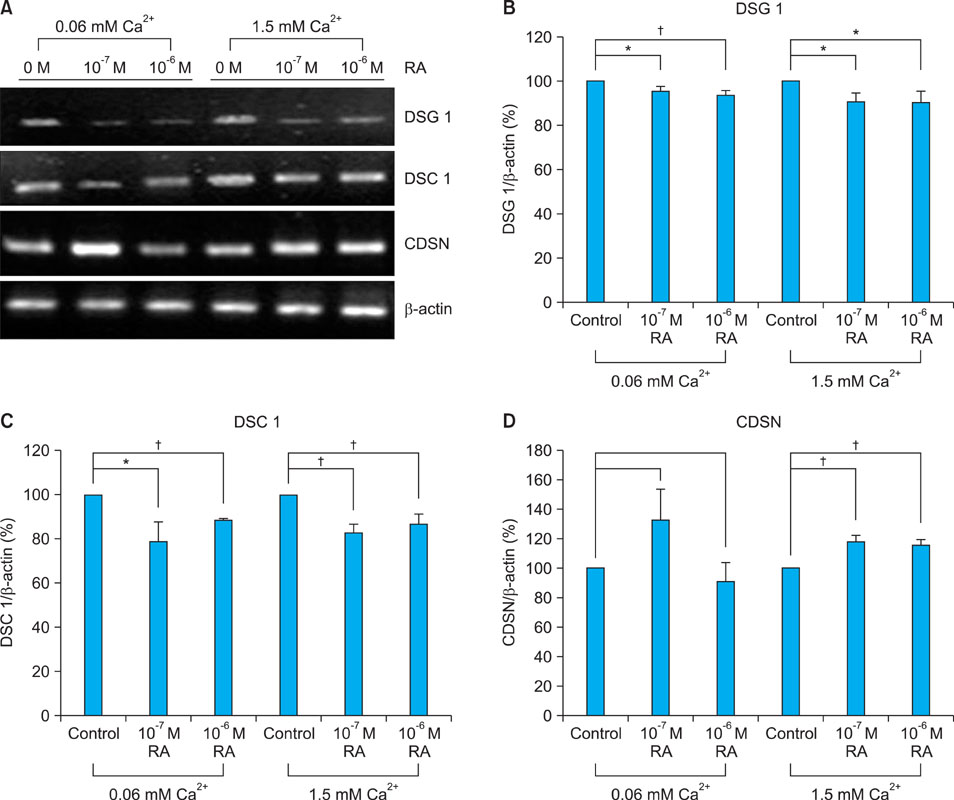
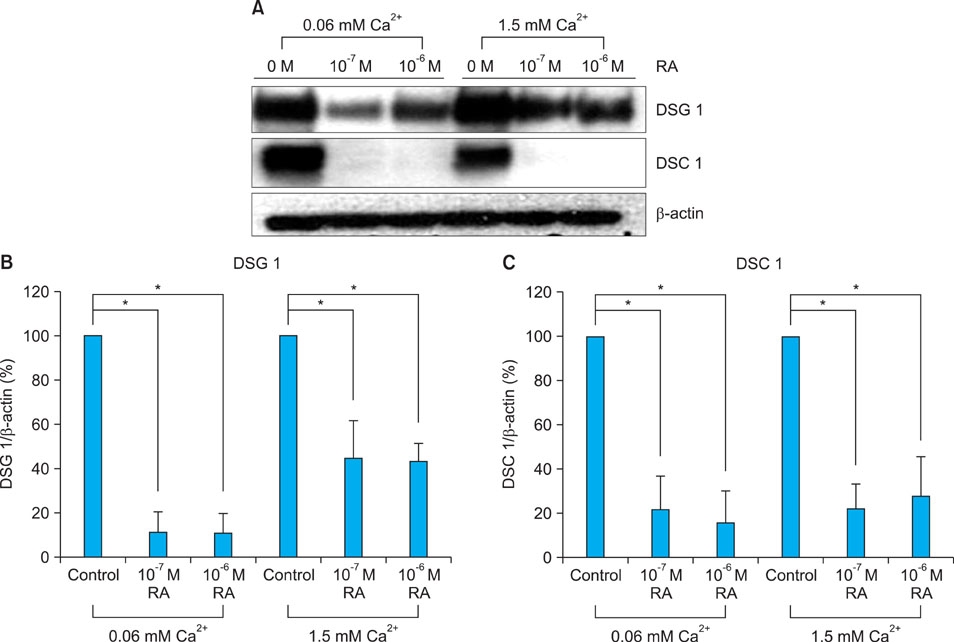
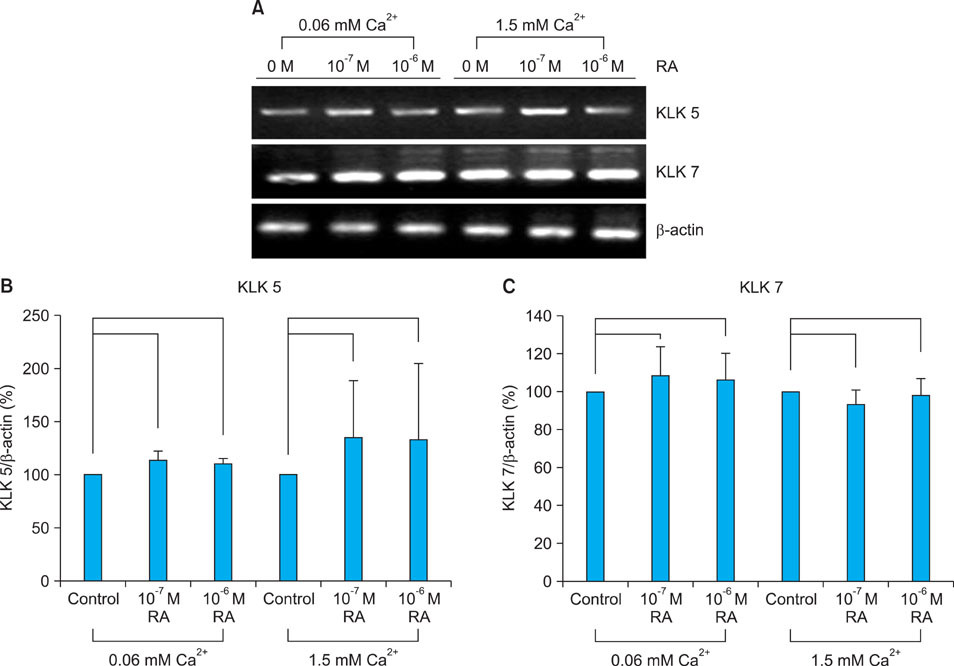
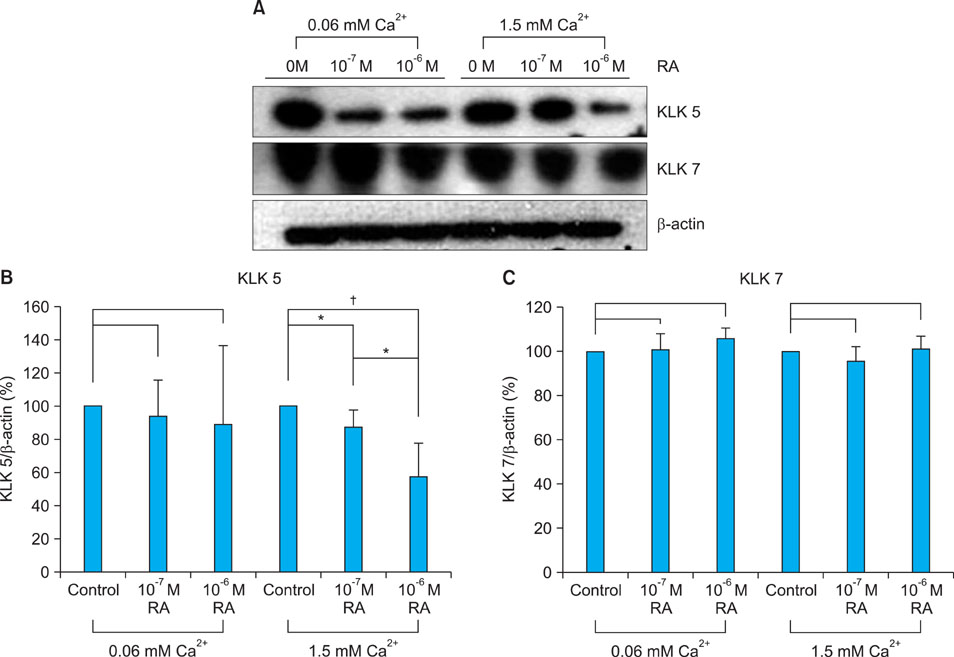
Topical retinol increased corneocyte detachment and degradation of corneodesmosomes. RA significantly decreased DSG1 and DSC1 expression at the mRNA and protein levels in keratinocytes that were cultured in both low- and high-calcium media. On the other hand, CDSN mRNA levels did not decrease in low-calcium media or increase in high-calcium media after RA treatment. KLK5 and KLK7 expression did not increase after RA treatment.
CONCLUSION
Our results indicate that DSG1 and DSC1 downregulation by RA could be related to the increased degradation of corneodesmosomes and consequent desquamation induced by retinoids.
Keyword
MeSH Terms
Figure
Reference
-
1. MacGregor JL, Maibach HI. The specificity of retinoid-induced irritation and its role in clinical efficacy. Exog Dermatol. 2002. 1:68–73.
Article2. Marks R, Hill S, Barton SP. The effects of an abrasive agent on normal skin and on photoaged skin in comparison with topical tretinoin. Br J Dermatol. 1990. 123:457–466.
Article3. Chen S, Ostrowski J, Whiting G, Roalsvig T, Hammer L, Currier SJ, et al. Retinoic acid receptor gamma mediates topical retinoid efficacy and irritation in animal models. J Invest Dermatol. 1995. 104:779–783.
Article4. Feng X, Peng ZH, Di W, Li XY, Rochette-Egly C, Chambon P, et al. Suprabasal expression of a dominant-negative RXR alpha mutant in transgenic mouse epidermis impairs regulation of gene transcription and basal keratinocyte proliferation by RAR-selective retinoids. Genes Dev. 1997. 11:59–71.
Article5. Effendy I, Weltfriend S, Patil S, Maibach HI. Differential irritant skin responses to topical retinoic acid and sodium lauryl sulphate: alone and in crossover design. Br J Dermatol. 1996. 134:424–430.
Article6. Ale SI, Laugier JP, Maibach HI. Differential irritant skin responses to tandem application of topical retinoic acid and sodium lauryl sulphate: II. Effect of time between first and second exposure. Br J Dermatol. 1997. 137:226–233.
Article7. Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981. 117:611–619.
Article8. Jonca N, Guerrin M, Hadjiolova K, Caubet C, Gallinaro H, Simon M, et al. Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J Biol Chem. 2002. 277:5024–5029.
Article9. Kowalczyk AP, Bornslaeger EA, Norvell SM, Palka HL, Green KJ. Desmosomes: intercellular adhesive junctions specialized for attachment of intermediate filaments. Int Rev Cytol. 1999. 185:237–302.
Article10. Serre G, Mils V, Haftek M, Vincent C, Croute F, Réano A, et al. Identification of late differentiation antigens of human cornified epithelia, expressed in re-organized desmosomes and bound to cross-linked envelope. J Invest Dermatol. 1991. 97:1061–1072.
Article11. Ekholm IE, Brattsand M, Egelrud T. Stratum corneum tryptic enzyme in normal epidermis: a missing link in the desquamation process? J Invest Dermatol. 2000. 114:56–63.12. Rawlings AV, Matts PJ. Stratum corneum moisturization at the molecular level: an update in relation to the dry skin cycle. J Invest Dermatol. 2005. 124:1099–1110.
Article13. Simon M, Jonca N, Guerrin M, Haftek M, Bernard D, Caubet C, et al. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J Biol Chem. 2001. 276:20292–20299.
Article14. Guerrin M, Simon M, Montézin M, Haftek M, Vincent C, Serre G. Expression cloning of human corneodesmosin proves its identity with the product of the S gene and allows improved characterization of its processing during keratinocyte differentiation. J Biol Chem. 1998. 273:22640–22267.
Article15. Levi K, Baxter J, Meldrum H, Misra M, Pashkovski E, Dauskardt RH. Effect of corneodesmosome degradation on the intercellular delamination of human stratum corneum. J Invest Dermatol. 2008. 128:2345–2347.
Article16. Hatakeyama S, Hayashi S, Yoshida Y, Otsubo A, Yoshimoto K, Oikawa Y, et al. Retinoic acid disintegrated desmosomes and hemidesmosomes in stratified oral keratinocytes. J Oral Pathol Med. 2004. 33:622–628.
Article17. Denning MF, Guy SG, Ellerbroek SM, Norvell SM, Kowalczyk AP, Green KJ. The expression of desmoglein isoforms in cultured human keratinocytes is regulated by calcium, serum, and protein kinase C. Exp Cell Res. 1998. 239:50–59.
Article18. McMullan R, Lax S, Robertson VH, Radford DJ, Broad S, Watt FM, et al. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol. 2003. 13:2185–2189.
Article19. Kim SC, Won JH. The effects of calcium and retinoic acid on epidermal desmosomes. Korean J Dermatol. 1994. 32:820–831.20. Peck GL, Elias PM, Wetzel B. Effects of retinoic acid on embryonic chick skin. J Invest Dermatol. 1977. 69:463–476.
Article21. Wanner R, Wolff B, Glowacki F, Kolde G, Wittig B. The loss of desmosomes after retinoic acid treatment results in an apparent inhibition of HaCaT keratinocyte differentiation. Arch Dermatol Res. 1999. 291:346–353.
Article22. Humphries JD, Parry EJ, Watson RE, Garrod DR, Griffiths CE. All-trans retinoic acid compromises desmosome expression in human epidermis. Br J Dermatol. 1998. 139:577–584.
Article23. Bernard FX, Pedretti N, Rosdy M, Deguercy A. Comparison of gene expression profiles in human keratinocyte monolayer cultures, reconstituted epidermis and normal human skin; transcriptional effects of retinoid treatments in reconstituted human epidermis. Exp Dermatol. 2002. 11:59–74.24. Simon M, Bernard D, Minondo AM, Camus C, Fiat F, Corcuff P, et al. Persistence of both peripheral and non-peripheral corneodesmosomes in the upper stratum corneum of winter xerosis skin versus only peripheral in normal skin. J Invest Dermatol. 2001. 116:23–30.
Article25. Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007. 127:2499–2515.
Article26. Dusek RL, Godsel LM, Green KJ. Discriminating roles of desmosomal cadherins: beyond desmosomal adhesion. J Dermatol Sci. 2007. 45:7–21.
Article27. Arnemann J, Sullivan KH, Magee AI, King IA, Buxton RS. Stratification-related expression of isoforms of the desmosomal cadherins in human epidermis. J Cell Sci. 1993. 104:741–750.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of PUVA and Retinoid-PUVA in the Treatment of Psoriasis in Korean Patients
- Retinoid Metabolism and Diabetes Mellitus
- The Therapeutic Effect of Oral Retinoid (Ro - 10 - 9359) on Psoriasis Vulgaris
- Alternating Topical Treatment with Perinoic Acid And Retinoid (Ro 11 - 1430) on Acne Vulgaris: therapeutic effect and side reactions
- The Efficacy and Sagety of Re-PUVA Combination Therapy for Psoriasis in Comparison with Retinoid and PUVA Monotherapy