Yonsei Med J.
2016 Sep;57(5):1282-1285. 10.3349/ymj.2016.57.5.1282.
Platelet-Rich Fibrin Lysate Can Ameliorate Dysfunction of Chronically UVA-Irradiated Human Dermal Fibroblasts
- Affiliations
-
- 1Department of Dermato-Venereology, Faculty of Medicine, Gadjah Mada University, Sardjito Hospital, Yogyakarta, Indonesia. widiokarsono@yahoo.com
- KMID: 2374177
- DOI: http://doi.org/10.3349/ymj.2016.57.5.1282
Abstract
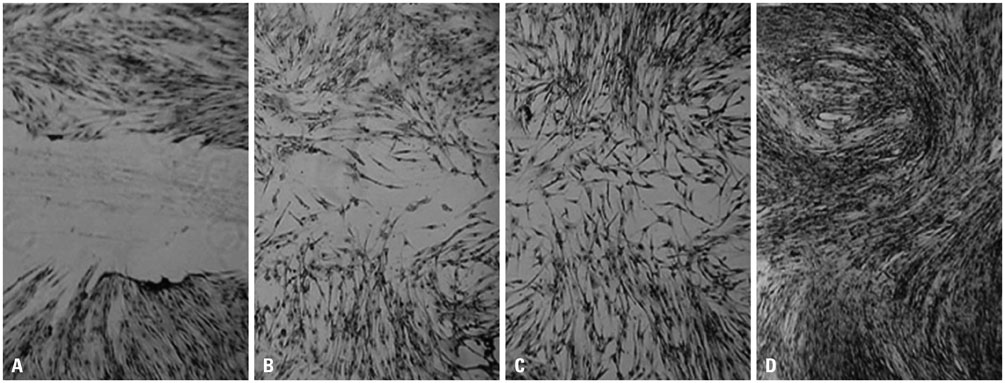
- To determine whether platelet-rich fibrin lysate (PRF-L) could restore the function of chronically ultraviolet-A (UVA)-irradiated human dermal fibroblasts (HDFs), we isolated and sub-cultured HDFs from six different human foreskins. HDFs were divided into two groups: those that received chronic UVA irradiation (total dosages of 10 J cm-2) and those that were not irradiated. We compared the proliferation rates, collagen deposition, and migration rates between the groups and between chronically UVA-irradiated HDFs in control and PRF-L-treated media. Our experiment showed that chronic UVA irradiation significantly decreased (p<0.05) the proliferation rates, migration rates, and collagen deposition of HDFs, compared to controls. Compared to control media, chronically UVA-irradiated HDFs in 50% PRF-L had significantly increased proliferation rates, migration rates, and collagen deposition (p<0.05), and the migration rates and collagen deposition of chronically UVA-irradiated HDFs in 50% PRF-L were equal to those of normal fibroblasts. Based on this experiment, we concluded that PRF-L is a good candidate material for treating UVA-induced photoaging of skin, although the best method for its clinical application remains to be determined.
MeSH Terms
Figure
Reference
-
1. Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence--implications for treatment. Int Wound J. 2005; 2:364–368.2. Clark RA. Oxidative stress and "senescent" fibroblasts in non-healing wounds as potential therapeutic targets. J Invest Dermatol. 2008; 128:2361–2364.
Article3. Margolis DJ, Morris LM, Papadopoulos M, Weinberg L, Filip JC, Lang SA, et al. Phase I study of H5.020CMV.PDGF-beta to treat venous leg ulcer disease. Mol Ther. 2009; 17:1822–1829.4. Czuwara-Ladykowska J, Gore EA, Shegogue DA, Smith EA, Trojanowska M. Differential regulation of transforming growth factor-beta receptors type I and II by platelet-derived growth factor in human dermal fibroblasts. Br J Dermatol. 2001; 145:569–575.
Article5. Pan DB, Ke YS, Liu WJ, Wei YQ, Tang J, Cao H. [Platelet-derived growth factor-BB inhibited p21(WAF1) expression partially through transforming growth factor-beta signalling system in vascular smooth muscle cell]. Zhonghua Xin Xue Guan Bing Za Zhi. 2010; 38:160–165.6. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004; 91:4–15.
Article7. Ficarelli E, Bernuzzi G, Tognetti E, Bussolati O, Zucchi A, Adorni D, et al. Treatment of chronic venous leg ulcers by platelet gel. Dermatol Ther. 2008; 21:Suppl 1. S13–S17.
Article8. Dionyssiou D, Demiri E, Foroglou P, Cheva A, Saratzis N, Aivazidis C, et al. The effectiveness of intralesional injection of platelet-rich plasma in accelerating the healing of chronic ulcers: an experimental and clinical study. Int Wound J. 2013; 10:397–406.
Article9. Yin B, Jiang X. Telomere shortening in cultured human dermal fibroblasts is associated with acute photodamage induced by UVA irradiation. Postepy Dermatol Alergol. 2013; 30:13–18.
Article10. Naru E, Suzuki T, Moriyama M, Inomata K, Hayashi A, Arakane K, et al. Functional changes induced by chronic UVA irradiation to cultured human dermal fibroblasts. Br J Dermatol. 2005; 153:Suppl 2. 6–12.
Article11. Schwartz E, Cruickshank FA, Christensen CC, Perlish JS, Lebwohl M. Collagen alterations in chronically sun-damaged human skin. Photochem Photobiol. 1993; 58:841–844.
Article12. Chung JH, Seo JY, Choi HR, Lee MK, Youn CS, Rhie G, et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001; 117:1218–1224.
Article13. Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004; 165:741–751.
Article14. Wlaschek M, Briviba K, Stricklin GP, Sies H, Scharffetter-Kochanek K. Singlet oxygen may mediate the ultraviolet A-induced synthesis of interstitial collagenase. J Invest Dermatol. 1995; 104:194–198.
Article15. Menter JM, Cornelison LM, Cannick L, Patta AM, Dowdy JC, Sayre RM, et al. Effect of UV on the susceptibility of acid-soluble Skh-1 hairless mouse collagen to collagenase. Photodermatol Photoimmunol Photomed. 2003; 19:28–34.
Article16. Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009; 27:63–69.
Article17. He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108:707–713.
Article18. Taskiran D, Taskiran E, Yercan H, Kutay FZ. Quantification of total collagen in rabbit tendon by the sirius red method. Tr J Med Sci. 1999; 29:7–9.19. Yarrow JC, Perlman ZE, Westwood NJ, Mitchison TJ. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004; 4:21.20. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004; 114:1502–1508.
Article21. Kim MS, Song HJ, Lee SH, Lee CK. Comparative study of various growth factors and cytokines on type I collagen and hyaluronan production in human dermal fibroblasts. J Cosmet Dermatol. 2014; 13:44–51.
Article22. Porsch H, Mehic´ M, Olofsson B, Heldin P, Heldin CH. Platelet-derived growth factor β-receptor, transforming growth factor β type I receptor, and CD44 protein modulate each other's signaling and stability. J Biol Chem. 2014; 289:19747–19757.
Article23. Villeneuve J, Block A, Le Bousse-Kerdilès MC, Lepreux S, Nurden P, Ripoche J, et al. Tissue inhibitors of matrix metalloproteinases in platelets and megakaryocytes: a novel organization for these secreted proteins. Exp Hematol. 2009; 37:849–856.
Article24. Lacarrubba F, Tedeschi A, Nardone B, Micali G. Mesotherapy for skin rejuvenation: assessment of the subepidermal low-echogenic band by ultrasound evaluation with cross-sectional B-mode scanning. Dermatol Ther. 2008; 21:Suppl 3. S1–S5.
Article25. Suswardana , Radiono S, Chusniyati N, Rosmelia , Wirohadidjojo YW. The effect of 50% glycolic acid on the percutaneous absorption of eutectic mixture of local anesthetics (EMLA): a study of the electrofulguration-induced pain. Int J Dermatol. 2008; 47:280–283.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of All-Trans-Retinoic Acid and Ursolic Acid on the Ultraviolet A Radiation Induced AP-1 (Fos/Jun) Activity in Cultured Human Dermal Fibroblasts
- Effect of Platelet-rich Plasma on Burn Wounds according to Time of Application: An Experimental Study on Rats
- The Anti-Diabetic Pinitol Improves Damaged Fibroblasts
- The Effects of Injectable Platelet-Rich Fibrin and AdvancedPlatelet Rich Fibrin on Gingival Fibroblast Cell Vitality, Proliferation, Differentiation
- Platelet rich fibrin - a novel acumen into regenerative endodontic therapy