Ann Clin Microbiol.
2017 Mar;20(1):7-12. 10.5145/ACM.2017.20.1.7.
Effect of Delayed Entry on Time to Detection for the Lactose Nonfermentative Gram-Negative Rods
- Affiliations
-
- 1Convergence of Medical Sciences, Gyeongsang National University School of Medicine, Jinju, Korea.
- 2Department of Laboratory Medicine, Gyeongsang Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea. sjkim8239@hanmail.net
- KMID: 2372999
- DOI: http://doi.org/10.5145/ACM.2017.20.1.7
Abstract
- BACKGROUND
Prolonged transport or poor accessibility of blood culture equipment during night time may cause delayed entry of blood culture bottles. The effect of prestorage conditions on time to detection (TTD) for the blood culture was evaluated for the important gram-negative lactose nonfermentative bacteria.
METHODS
Three different clinical isolates of Pseudomonas aeruginosa, Acinetobacter baumannii, Stenotrophomonas maltophilia, and Burkholdera cepacia were diluted to 150 CFU/mL and 15 CFU/mL and inoculated into standard aerobic bottles. These were stored at 25℃ and at 37℃ for 0, 6, 12, 18, and 24 h. They were entered to BacT/Alert 3D Systems (bio-Mérieux Inc.) and TTD was monitored for each condition.
RESULTS
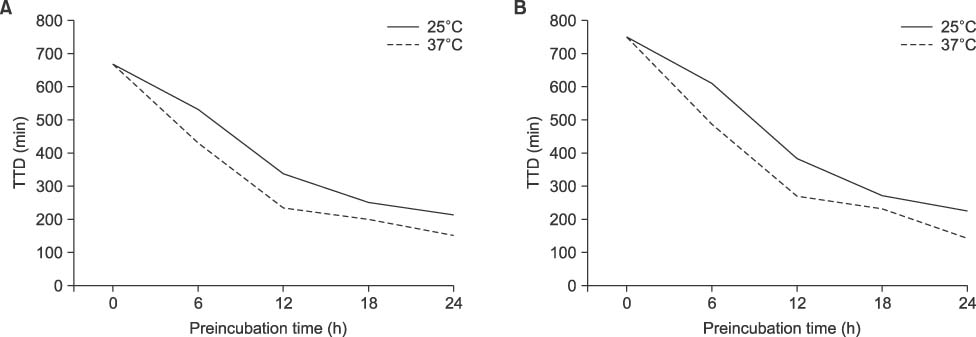
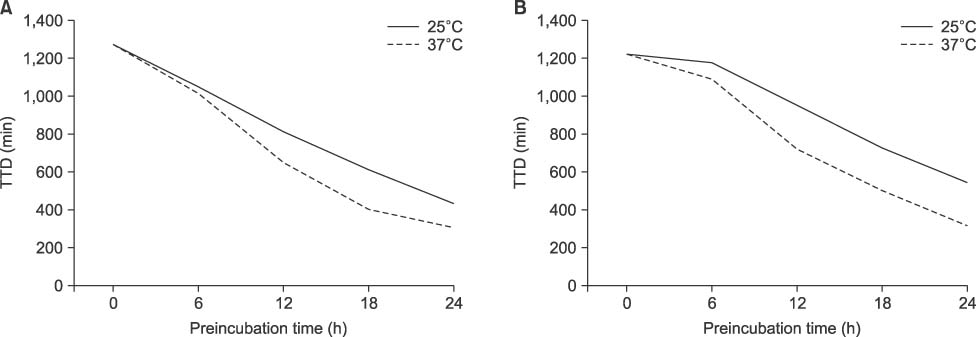
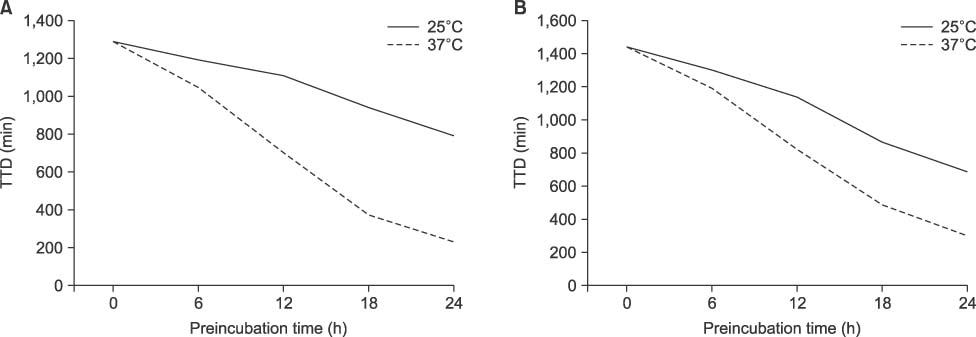
At the 150 CFU/mL concentration, P. aeruginosa and A. baumannii showed false-negative for the bottles prestored at 37℃ for 18 h and 24 h, respectively. However, there was no false-negative for S. maltophilia or B. cepacia at any prestorage conditions. There was a significant decrease of TTD for all experimental microorganisms except P. aeruginosa prestored for 24 h either at 25℃ or at 37℃ (P< 0.05).
CONCLUSION
Delayed entry may cause false-negative, especially for the high level of bacteremia of P. aeruginosa or A. baumannii when the bottles are stored at 37℃ for ≥18 h. TTD could be reduced by prestorage of the bottles at 37℃ until 12 h without false-negative for nonfermentative bacteria.
Keyword
MeSH Terms
Figure
Reference
-
1. CLSI. Principles and procedures for blood cultures; approved guidline. CLSI document M47-A. Wayne, PA: Clinical and Laboratory Standards Institute;2007.2. van der Velden LB, Vos FJ, Mouton JW, Sturm PD. Clinical impact of preincubation of blood cultures at 37℃. J Clin Microbiol. 2011; 49:275–280.3. Kerremans JJ, van der Bij AK, Goessens W, Verbrugh HA, Vos MC. Immediate incubation of blood cultures outside routine laboratory hours of operation accelerates antibiotic switching. J Clin Microbiol. 2009; 47:3520–3523.4. Kim JS, Seok H, Kim S. Effect of preincubation of blood culture bottles in a BacT/Alert unit outside laboratory operating hours on detection time. Ann Clin Microbiol. 2014; 17:105–109.5. Klaerner HG, Eschenbach U, Kamereck K, Lehn N, Wagner H, Miethke T. Failure of an automated blood culture system to detect nonfermentative gram-negative bacteria. J Clin Microbiol. 2000; 38:1036–1041.6. Lemming L, Holt HM, Petersen IS, Østergaard C, Bruun B. Bactec 9240 blood culture system: to preincubate at 35 degrees C or not? Clin Microbiol Infect. 2004; 10:1089–1091.7. Ziegler R, Johnscher I, Martus P, Lenhardt D, Just HM. Controlled clinical laboratory comparison of two supplemented aerobic and anaerobic media used in automated blood culture systems to detect bloodstream infections. J Clin Microbiol. 1998; 36:657–661.8. Shigei JT, Shimabukuro JA, Pezzlo MT, de la Maza LM, Peterson EM. Value of terminal subcultures for blood cultures monitored by BACTEC 9240. J Clin Microbiol. 1995; 33:1385–1388.9. Chapin K, Lauderdale TL. Comparison of Bactec 9240 and Difco ESP blood culture systems for detection of organisms from vials whose entry was delayed. J Clin Microbiol. 1996; 34:543–549.10. Akan OA, Yildiz E. Comparison of the effect of delayed entry into 2 different blood culture systems (BACTEC 9240 and BacT/ALERT 3D) on culture positivity. Diagn Microbiol Infect Dis. 2006; 54:193–196.11. Seegmüller I, Eschenbach U, Kamereck K, Miethke T. Sensitivity of the BacT/ALERT FA-medium for detection of Pseudomonas aeruginosa in pre-incubated blood cultures and its temperature-dependence. J Med Microbiol. 2004; 53:869–874.12. Sautter RL, Bills AR, Lang DL, Ruschell G, Heiter BJ, Bourbeau PP. Effects of delayed-entry conditions on the recovery and detection of microorganisms from BacT/ALERT and BACTEC blood culture bottles. J Clin Microbiol. 2006; 44:1245–1249.13. Lee DH, Koh EH, Choi SR, Kim S, Kim DH, Lee NY. Effect of sodium citrate on growth of bacteria in blood culture. Ann Clin Microbiol. 2013; 16:168–173.14. Lee DH, Koh EH, Choi SR, Kim S. Growth dynamics of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa as a function of time to detection in BacT/Alert 3D blood culture bottles with various preincubation conditions. Ann Lab Med. 2013; 33:406–409.15. Forbes BA, Sahm DF, Weissfeld AS, editors. Study guide for Bailey & Scott's diagnostic microbiology. 12th ed. Philadelphia: Mosby Inc.;2007. p. 873–889.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification System of Nonfermentative Gram Negative Bacilli Using Microplate
- Calculation of Minimal Inhibitory Concentrations of Carbapenem from Zone Diameters of Disk Diffusion Test of Carbapenem-Resistant Nonfermentative Gram-Negative Bacilli
- A Numerical Coding System(MCRCODE-N) for Identification of Glucose Nonfermenting Gram-Negative Bacilli
- Lactose Intolerance and Colorectal Cancer
- In Vitro Antimicrobial Activities of NanoSilver-coated Gauze against Clinical Isolates