J Korean Med Sci.
2016 Feb;31(2):247-253. 10.3346/jkms.2016.31.2.247.
Clinical Characteristics and Factors Influencing the Occurrence of Acute Eosinophilic Pneumonia in Korean Military Personnel
- Affiliations
-
- 1Department of Preventive Medicine, The Armed Forces Medical Command, Seongnam, Korea.
- 2Division of Pulmonary and Critical Care Medicine, Department of Medicine, The Armed Forces Medical Command, Seongnam, Korea. byungwoo.jhun@gmail.com
- KMID: 2360049
- DOI: http://doi.org/10.3346/jkms.2016.31.2.247
Abstract
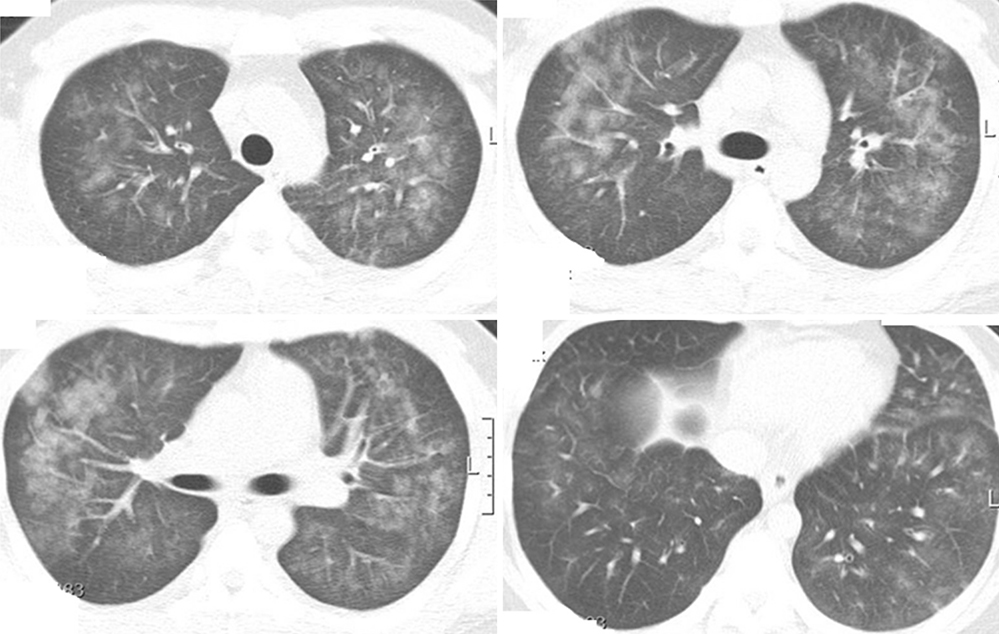
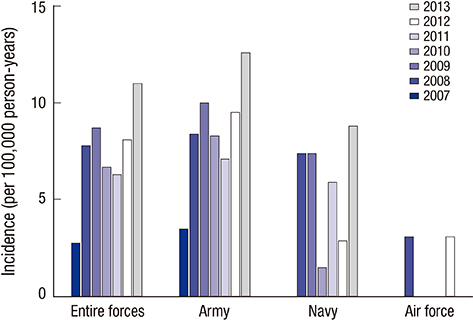
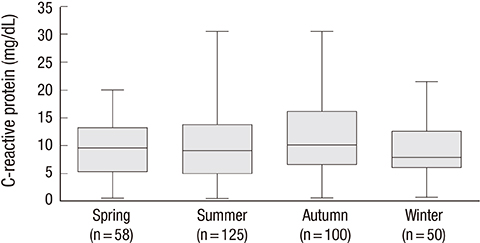
- Acute eosinophilic pneumonia (AEP) is an uncommon inflammatory lung disease, and limited data exist concerning the clinical characteristics and factors that influence its occurrence. We retrospectively reviewed the records of AEP patients treated at Korean military hospitals between January 2007 and December 2013. In total, 333 patients were identified; their median age was 22 years, and all were men. All patients presented with acute respiratory symptoms (cough, sputum, dyspnea, or fever) and had elevated levels of inflammatory markers including median values of 13,185/microL for white blood cell count and 9.51 mg/dL for C-reactive protein. All patients showed diffuse ground glass opacity/consolidation, and most had pleural effusion (n = 265; 80%) or interlobular septal thickening (n = 265; 85%) on chest computed tomography. Most patients had normal body mass index (n = 255; 77%), and only 30 (9%) patients had underlying diseases including rhinitis, asthma, or atopic dermatitis. Most patients had recently changed smoking habits (n = 288; 87%) and were Army personnel (n = 297; 89%).The AEP incidence was higher in the Army group compared to the Navy or Air Force group for every year (P = 0.002). Both the number of patients and patients with high illness severity (oxygen requirement, intensive care unit admission, and pneumonia severity score class > or = III) tended to increase as seasonal temperatures rose. We describe the clinical characteristics of AEP and demonstrate that AEP patients have recently changed smoking habits and work for the Army. There is an increasing tendency in the numbers of patients and those with higher AEP severity with rising seasonal temperatures.
MeSH Terms
-
Acute Disease
Asian Continental Ancestry Group
C-Reactive Protein/analysis
Cough/etiology
Dyspnea/etiology
Fever/etiology
Humans
Incidence
Leukocyte Count
Male
Military Personnel
Pleural Effusion/complications/diagnosis/radiography
Pulmonary Eosinophilia/complications/*diagnosis/pathology
Republic of Korea/epidemiology
Retrospective Studies
Seasons
Severity of Illness Index
Smoking
Tomography, X-Ray Computed
Young Adult
C-Reactive Protein
Figure
Reference
-
1. Allen JN, Pacht ER, Gadek JE, Davis WB. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989; 321:569–574.2. Philit F, Etienne-Mastroïanni B, Parrot A, Guérin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med. 2002; 166:1235–1239.3. Hayakawa H, Sato A, Toyoshima M, Imokawa S, Taniguchi M. A clinical study of idiopathic eosinophilic pneumonia. Chest. 1994; 105:1462–1466.4. Daimon T, Johkoh T, Sumikawa H, Honda O, Fujimoto K, Koga T, Arakawa H, Yanagawa M, Inoue A, Mihara N, et al. Acute eosinophilic pneumonia: thin-section CT findings in 29 patients. Eur J Radiol. 2008; 65:462–467.5. Rhee CK, Min KH, Yim NY, Lee JE, Lee NR, Chung MP, Jeon K. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013; 41:402–409.6. Jhun BW, Kim SJ, Kim K, Lee JE. Clinical implications of initial peripheral eosinophilia in acute eosinophilic pneumonia. Respirology. 2014; 19:1059–1065.7. Lim SY, Suh GY, Jeon K. Acute eosinophilic pneumonia presenting as life-threatening hypoxaemia necessitating extracorporeal membrane oxygenation. Int J Tuberc Lung Dis. 2012; 16:1711–1712.8. Kawayama T, Fujiki R, Morimitsu Y, Rikimaru T, Aizawa H. Fatal idiopathic acute eosinophilic pneumonia with acute lung injury. Respirology. 2002; 7:373–375.9. Imokawa S, Sato A, Hayakawa H, Toyoshima M, Taniguchi M, Chida K. Possible involvement of an environmental agent in the development of acute eosinophilic pneumonia. Ann Allergy Asthma Immunol. 1996; 76:419–422.10. Rom WN, Weiden M, Garcia R, Yie TA, Vathesatogkit P, Tse DB, McGuinness G, Roggli V, Prezant D. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am J Respir Crit Care Med. 2002; 166:797–800.11. Watanabe K, Fujimura M, Kasahara K, Yasui M, Myou S, Kita T, Watanabe A, Nakao S. Acute eosinophilic pneumonia following cigarette smoking: a case report including cigarette-smoking challenge test. Intern Med. 2002; 41:1016–1020.12. Uchiyama H, Suda T, Nakamura Y, Shirai M, Gemma H, Shirai T, Toyoshima M, Imokawa S, Yasuda K, Ida M, et al. Alterations in smoking habits are associated with acute eosinophilic pneumonia. Chest. 2008; 133:1174–1180.13. Kitahara Y, Matsumoto K, Taooka Y, Moritani C, Nakamura K, Ohashi N, Daido K, Arita K. Cigarette smoking-induced acute eosinophilic pneumonia showing tolerance in broncho-alveolar lavage findings. Intern Med. 2003; 42:1016–1021.14. Shiota Y, Kawai T, Matsumoto H, Hiyama J, Tokuda Y, Marukawa M, Ono T, Mashiba H. Acute eosinophilic pneumonia following cigarette smoking. Intern Med. 2000; 39:830–833.15. Katoh S, Matsumoto N, Matsumoto K, Fukushima K, Matsukura S. Elevated interleukin-18 levels in bronchoalveolar lavage fluid of patients with eosinophilic pneumonia. Allergy. 2004; 59:850–856.16. Mato N, Bando M, Kusano A, Hirano T, Nakayama M, Uto T, Nakaya T, Yamasawa H, Sugiyama Y. Clinical significance of interleukin 33 (IL-33) in patients with eosinophilic pneumonia. Allergol Int. 2013; 62:45–52.17. Miyazaki E, Nureki S, Fukami T, Shigenaga T, Ando M, Ito K, Ando H, Sugisaki K, Kumamoto T, Tsuda T. Elevated levels of thymus- and activation-regulated chemokine in bronchoalveolar lavage fluid from patients with eosinophilic pneumonia. Am J Respir Crit Care Med. 2002; 165:1125–1131.18. Allen JN, Liao Z, Wewers MD, Altenberger EA, Moore SA, Allen ED. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J Allergy Clin Immunol. 1996; 97:1366–1374.19. Okubo Y, Horie S, Hachiya T, Momose T, Tsukadaira A, Takashi S, Suzuki J, Isobe M, Sekiguchi M. Predominant implication of IL-5 in acute eosinophilic pneumonia: comparison with chronic eosinophilic pneumonia. Int Arch Allergy Immunol. 1998; 116:76–80.20. Lee JE, Rhee CK, Lim JH, Lee SM, Shim YS, Lee CT, Lee SW. Fraction of exhaled nitric oxide in patients with acute eosinophilic pneumonia. Chest. 2012; 141:1267–1272.21. Jhun BW, Kim SJ, Kim K, Lee JE, Hong DJ. Clinical implications of correlation between peripheral eosinophil count and serum levels of IL-5 and tryptase in acute eosinophilic pneumonia. Respir Med. 2014; 108:1655–1662.22. Shorr AF, Scoville SL, Cersovsky SB, Shanks GD, Ockenhouse CF, Smoak BL, Carr WW, Petruccelli BP. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA. 2004; 292:2997–3005.23. Matsuno O, Takenaka R, Hiroshige S, Ono E, Nishitake T, Ueno T, Miyazaki E, Kumamoto T. Reduced IgG levels found during acute eosinophilic pneumonia, which normalize during recovery from disease. Respir Med. 2008; 102:899–903.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Severe Adenovirus Pneumonia
- A Case of Acute eosinophilic pneumonia
- Vitamin D Status in South Korean Military Personnel with Acute Eosinophilic Pneumonia: A Pilot Study
- A case of acute eosinophilic pneumonia
- Cigarette Smoking-Induced Acute Eosinophilic Pneumonia: A Case Report Including a Provocation Test