J Pathol Transl Med.
2016 Sep;50(5):377-384. 10.4132/jptm.2016.07.25.
Do Helper T Cell Subtypes in Lymphocytic Thyroiditis Play a Role in the Antitumor Effect?
- Affiliations
-
- 1Department of Medicine, Yonsei University Graduate School, Seoul, Korea.
- 2Department of Laboratory Medicine, Chosun University College of Medicine, Gwangju, Korea.
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Microbiology, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of General Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Pathology, Sungkyunkwan University College of Medicine, Seoul, Korea.
- 7Department of Pathology, Rehabilitation Institute of Neuromuscular Disease, Yonsei University College of Medicine, Seoul, Korea. soonwonh@yuhs.ac
- KMID: 2353600
- DOI: http://doi.org/10.4132/jptm.2016.07.25
Abstract
- BACKGROUND
Papillary thyroid carcinoma (PTC) is frequently accompanied by lymphocytic thyroiditis (LT). Some reports claim that Hashimoto's thyroiditis (the clinical form of LT) enhances the likelihood of PTC; however, others suggest that LT has antitumor activity. This study was aimed to find out the relationship between the patterns of helper T cell (Th) cytokines in thyroid tissue of PTC with or without LT and the clinicopathological manifestation of PTC.
METHODS
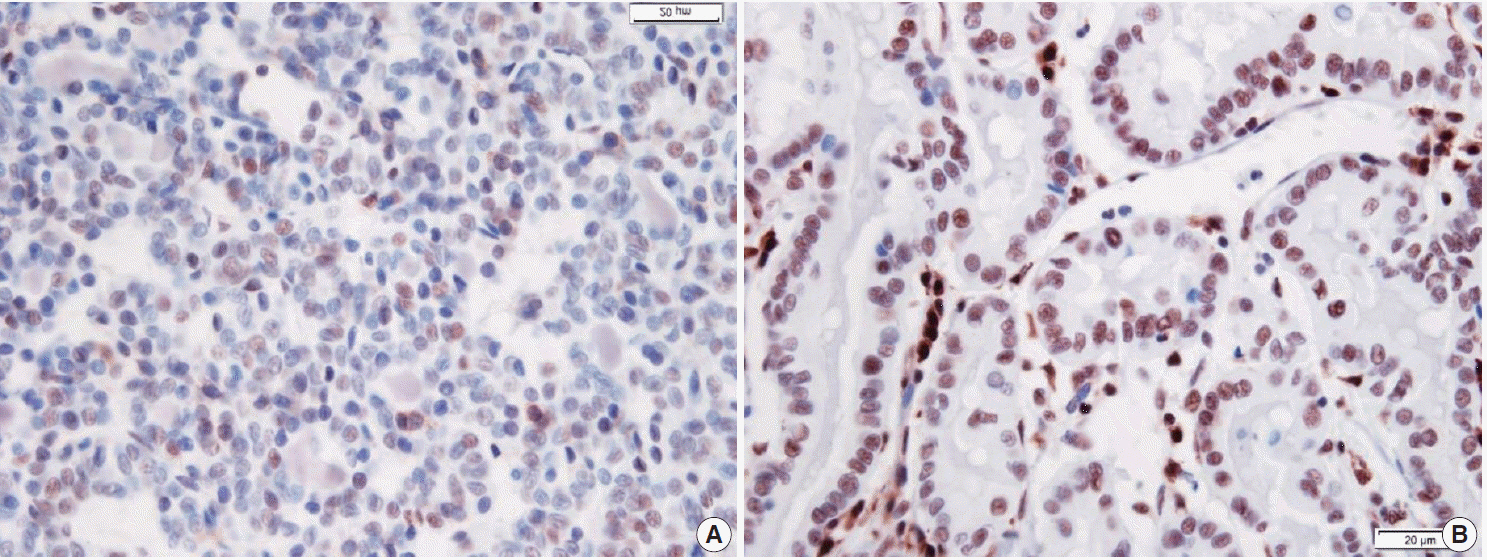
Fresh surgical samples of PTC with (13 cases) or without (10 cases) LT were used. The prognostic parameters (tumor size, extra-thyroidal extension of PTC, and lymph node metastasis) were analyzed. The mRNA levels of two subtypes of Th cytokines, Th1 (tumor necrosis factor α [TNF-α], interferon γ [IFN-γ ], and interleukin [IL] 2) and Th2 (IL-4 and IL-10), were analyzed. Because most PTC cases were microcarcinomas and recent cases without clinical follow-up, negative or faint p27 immunoreactivity was used as a surrogate marker for lymph node metastasis.
RESULTS
PTC with LT cases showed significantly higher expression of TNF-α (p = .043), IFN-γ (p < .010), IL-4 (p = .015) than those without LT cases. Although the data were not statistically significant, all analyzed cytokines (except for IL-4) were highly expressed in the cases with higher expression of p27 surrogate marker.
CONCLUSIONS
These results indicate that mixed Th1 (TNF-α, IFN-γ , and IL-2) and Th2 (IL-10) immunity might play a role in the antitumor effect in terms of lymph node metastasis.
Keyword
MeSH Terms
-
Biomarkers
Cyclin-Dependent Kinase Inhibitor p27
Cytokines
Follow-Up Studies
Interferons
Interleukin-4
Interleukins
Lymph Nodes
Necrosis
Neoplasm Metastasis
RNA, Messenger
T-Lymphocytes, Helper-Inducer
Thyroid Gland
Thyroid Neoplasms
Thyroiditis
Thyroiditis, Autoimmune*
Biomarkers
Cyclin-Dependent Kinase Inhibitor p27
Cytokines
Interferons
Interleukin-4
Interleukins
RNA, Messenger
Figure
Reference
-
1. Kuijpens JL, Coebergh JW, van der Heijden LH, Kruis H, Ribot JG, de Rooij HA. Thyroid cancer in Southeastern Netherlands, 1970-1989: trends in incidence, treatment and survival. Ned Tijdschr Geneeskd. 1994; 138:464–8.2. Laurberg P, Nøhr SB, Pedersen KM, et al. Thyroid disorders in mild iodine deficiency. Thyroid. 2000; 10:951–63.
Article3. Ministry for Health, Welfare and Family Affairs; Korea Central Cancer Registry. Cancer incidence in Korea 1999-2002. Goyang: Korea Central Cancer Registry;2008.4. Sobrinho-Simoes M, Preto A, Rocha AS, et al. Molecular pathology of well-differentiated thyroid carcinomas. Virchows Arch. 2005; 447:787–93.
Article5. Siironen P, Louhimo J, Nordling S, et al. Prognostic factors in papillary thyroid cancer: an evaluation of 601 consecutive patients. Tumour Biol. 2005; 26:57–64.
Article6. Shibru D, Chung KW, Kebebew E. Recent developments in the clinical application of thyroid cancer biomarkers. Curr Opin Oncol. 2008; 20:13–8.
Article7. Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007; 246:466–70.8. Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A. Lessons from randomized phase III studies with active cancer immunotherapies--outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC). Vaccine. 2007; 25 Suppl 2:B97–109.9. McConahey WM, Hay ID, Woolner LB, van Heerden JA, Taylor WF. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1986; 61:978–96.
Article10. Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999; 126:1070–6.
Article11. Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007; 70:1–11.
Article12. Mardente S, Lenti L, Lococo E, et al. Phenotypic and functional characterization of lymphocytes in autoimmune thyroiditis and in papillary carcinoma. Anticancer Res. 2005; 25:2483–8.13. Baker JR Jr, Fosso CK. Immunological aspects of cancers arising from thyroid follicular cells. Endocr Rev. 1993; 14:729–46.
Article14. Shull JH, Sharon N, Victor TA, Scanlon EF. Thyroid carcinoma: immunology, irradiation, and lymphocytic infiltration. Arch Surg. 1979; 114:729–31.15. Mauras N, Zimmerman D, Goellner JR. Hashimoto thyroiditis associated with thyroid cancer in adolescent patients. J Pediatr. 1985; 106:895–8.
Article16. Amino N, Pysher T, Cohen EP, Degroot LJ. Immunologic aspects of human thyroid cancer: humoral and cell-mediated immunity, and a trial of immunotherapy. Cancer. 1975; 36:963–73.17. Gerfo PL, Feind C, Weber C, Ting W. Immunotherapy of thyroid cancer by induction of autoimmune thyroiditis. Surgery. 1983; 94:959–65.18. Karlidag T, Cobanoglu B, Keles E, et al. Expression of Bax, p53, and p27/kip in patients with papillary thyroid carcinoma with or without cervical nodal metastasis. Am J Otolaryngol. 2007; 28:31–6.
Article19. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29:e45.
Article20. Resnick MB, Schacter P, Finkelstein Y, Kellner Y, Cohen O. Immunohistochemical analysis of p27/kip1 expression in thyroid carcinoma. Mod Pathol. 1998; 11:735–9.21. Vella V, Mineo R, Frasca F, et al. Interleukin-4 stimulates papillary thyroid cancer cell survival: implications in patients with thyroid cancer and concomitant Graves’ disease. J Clin Endocrinol Metab. 2004; 89:2880–9.
Article22. Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto’s thyroiditis (Th1) and Graves’ disease (Th2). Neuroimmunomodulation. 2004; 11:209–13.
Article23. Ajjan RA, Watson PF, McIntosh RS, Weetman AP. Intrathyroidal cytokine gene expression in Hashimoto’s thyroiditis. Clin Exp Immunol. 1996; 105:523–8.
Article24. Yip I, Pang XP, Berg L, Hershman JM. Antitumor actions of interferon-gamma and interleukin-1 beta on human papillary thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1995; 80:1664–9.
Article25. Paulson LM, Shindo ML, Schuff KG. Role of chronic lymphocytic thyroiditis in central node metastasis of papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2012; 147:444–9.
Article26. Mitsiades CS, Poulaki V, Mitsiades N. The role of apoptosis-inducing receptors of the tumor necrosis factor family in thyroid cancer. J Endocrinol. 2003; 178:205–16.
Article27. Ahn D, Heo SJ, Park JH, et al. Clinical relationship between Hashimoto’s thyroiditis and papillary thyroid cancer. Acta Oncol. 2011; 50:1228–34.
Article28. Yoon YH, Kim HJ, Lee JW, Kim JM, Koo BS. The clinicopathologic differences in papillary thyroid carcinoma with or without co-existing chronic lymphocytic thyroiditis. Eur Arch Otorhinolaryngol. 2012; 269:1013–7.
Article29. Boi F, Lai ML, Marziani B, Minerba L, Faa G, Mariotti S. High prevalence of suspicious cytology in thyroid nodules associated with positive thyroid autoantibodies. Eur J Endocrinol. 2005; 153:637–42.
Article30. Lucas SD, Karlsson-Parra A, Nilsson B, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol. 1996; 27:1329–35.
Article31. Larrea MD, Liang J, Da Silva T, et al. Phosphorylation of p27Kip1 regulates assembly and activation of cyclin D1-Cdk4. Mol Cell Biol. 2008; 28:6462–72.32. Lee J, Kim SS. The function of p27 KIP1 during tumor development. Exp Mol Med. 2009; 41:765–71.33. Khoo ML, Beasley NJ, Ezzat S, Freeman JL, Asa SL. Overexpression of cyclin D1 and underexpression of p27 predict lymph node metastases in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2002; 87:1814–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Antitumor Mechanism of Intravesical BCG Therapy in Tumorigenesis(I): Study About T Cell Dependency
- A case of Hashimoto's thyroiditis with chronic lymphocytic leukemia
- The Role of STAT1 in T Helper Cell Differentiation during Breast Cancer Progression
- Lymphocytic Thyroiditis Presenting as a Focal Uptake on 18F-Fluorodeoxyglucose Positron Emission Tomography: A Case Report
- Lymphocytic Thyroiditis Presenting as Diffuse Microcalcifications on Ultrasound: A Case Report