J Gynecol Oncol.
2015 Oct;26(4):249-251. 10.3802/jgo.2015.26.4.249.
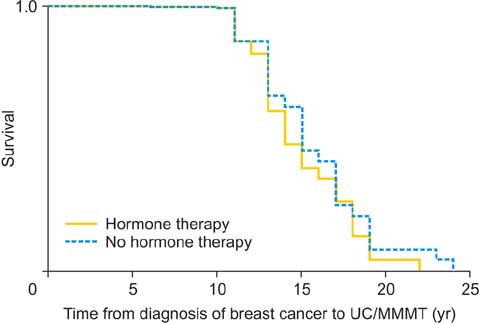
Uterine carcinosarcoma/malignant mixed Mullerian tumor incidence is increased in women with breast cancer, but independent of hormone therapy
- Affiliations
-
- 1Institute of Genetic Medicine, Newcastle University, International Centre for Life, Newcastle upon Tyne, UK. brian.wilson@ncl.ac.uk
- 2Northern Genetics Service, Newcastle upon Tyne Hospitals NHS Foundation Trust, International Centre for Life, Newcastle upon Tyne, UK.
- KMID: 2345913
- DOI: http://doi.org/10.3802/jgo.2015.26.4.249
Abstract
- No abstract available.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Breast Neoplasms/*epidemiology/genetics
Carcinosarcoma/*epidemiology/genetics
Cohort Studies
England/epidemiology
Female
Genes, BRCA1
Genes, BRCA2
Hormone Replacement Therapy/*statistics & numerical data
Humans
Incidence
Kaplan-Meier Estimate
Middle Aged
Mixed Tumor, Mullerian/*epidemiology/genetics
Mutation/genetics
Neoplasms, Second Primary/*epidemiology
Uterine Neoplasms/*epidemiology/genetics
Figure
Reference
-
1. Kernochan LE, Garcia RL. Carcinosarcomas (malignant mixed Müllerian tumor) of the uterus: advances in elucidation of biologic and clinical characteristics. J Natl Compr Canc Netw. 2009; 7:550–556.2. Ho SP, Ho TH. Malignant mixed Mullerian tumours of the uterus: a ten-year experience. Singapore Med J. 2002; 43:452–456.3. Friedrich M, Villena-Heinsen C, Mink D, Bonkhoff H, Schmidt W. Carcinosarcoma, endometrial intraepithelial carcinoma and endometriosis after tamoxifen therapy in breast cancer. Eur J Obstet Gynecol Reprod Biol. 1999; 82:85–87.4. Fotiou S, Hatjieleftheriou G, Kyrousis G, Kokka F, Apostolikas N. Long-term tamoxifen treatment: a possible aetiological factor in the development of uterine carcinosarcoma: two case-reports and review of the literature. Anticancer Res. 2000; 20:2015–2020.5. McCluggage WG, Abdulkader M, Price JH, Kelehan P, Hamilton S, Beattie J, et al. Uterine carcinosarcomas in patients receiving tamoxifen: a report of 19 cases. Int J Gynecol Cancer. 2000; 10:280–284.6. Kloos I, Delaloge S, Pautier P, Di Palma M, Goupil A, Duvillard P, et al. Tamoxifen-related uterine carcinosarcomas occur under/after prolonged treatment: report of five cases and review of the literature. Int J Gynecol Cancer. 2002; 12:496–500.7. Hubalek M, Ramoni A, Mueller-Holzner E, Marth C. Malignant mixed mesodermal tumor after tamoxifen therapy for breast cancer. Gynecol Oncol. 2004; 95:264–266.8. Yildirim Y, Inal MM, Sanci M, Yildirim YK, Mit T, Polat M, et al. Development of uterine sarcoma after tamoxifen treatment for breast cancer: report of four cases. Int J Gynecol Cancer. 2005; 15:1239–1242.9. Arenas M, Rovirosa A, Hernandez V, Ordi J, Jorcano S, Mellado B, et al. Uterine sarcomas in breast cancer patients treated with tamoxifen. Int J Gynecol Cancer. 2006; 16:861–865.10. Curtis RE, Freedman DM, Sherman ME, Fraumeni JF Jr. Risk of malignant mixed mullerian tumors after tamoxifen therapy for breast cancer. J Natl Cancer Inst. 2004; 96:70–74.11. Rieck GC, Freites ON, Williams S. Is tamoxifen associated with high-risk endometrial carcinomas? A retrospective case series of 196 women with endometrial cancer. J Obstet Gynaecol. 2005; 25:39–41.12. Swerdlow AJ, Jones ME. British Tamoxifen Second Cancer Study Group. Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005; 97:375–384.13. Saadat M, Truong PT, Kader HA, Speers CH, Berthelet E, McMurtrie E, et al. Outcomes in patients with primary breast cancer and a subsequent diagnosis of endometrial cancer: comparison of cohorts treated with and without tamoxifen. Cancer. 2007; 110:31–37.14. Watanabe T, Inoue S, Ogawa S, Ishii Y, Hiroi H, Ikeda K, et al. Agonistic effect of tamoxifen is dependent on cell type, ERE-promoter context, and estrogen receptor subtype: functional difference between estrogen receptors alpha and beta. Biochem Biophys Res Commun. 1997; 236:140–145.15. Zafrakas M, Kostopoulou E, Dragoumis K, Mikos T, Papadimas J, Bontis J. Expression of estrogen receptors alpha and beta in two uterine mesenchymal tumors after prolonged tamoxifen therapy: report of two cases. Eur J Gynaecol Oncol. 2004; 25:530–533.16. Raspollini MR, Mecacci F, Paglierani M, Marchionni M, Taddei GL. HER-2/neu oncogene in uterine carcinosarcoma on tamoxifen therapy. Pathol Res Pract. 2005; 201:141–144.17. Amant F, Vloeberghs V, Woestenborghs H, Debiec-Rychter M, Verbist L, Moerman P, et al. ERBB-2 gene overexpression and amplification in uterine sarcomas. Gynecol Oncol. 2004; 95:583–587.18. Marques MM, Beland FA. Identification of tamoxifen-DNA adducts formed by 4-hydroxytamoxifen quinone methide. Carcinogenesis. 1997; 18:1949–1954.19. Martin EA, Brown K, Gaskell M, Al-Azzawi F, Garner RC, Boocock DJ, et al. Tamoxifen DNA damage detected in human endometrium using accelerator mass spectrometry. Cancer Res. 2003; 63:8461–8465.20. Segev Y, Iqbal J, Lubinski J, Gronwald J, Lynch HT, Moller P, et al. The incidence of endometrial cancer in women with BRCA1 and BRCA2 mutations: an international prospective cohort study. Gynecol Oncol. 2013; 130:127–131.21. Pennington KP, Walsh T, Lee M, Pennil C, Novetsky AP, Agnew KJ, et al. BRCA1, TP53, and CHEK2 germline mutations in uterine serous carcinoma. Cancer. 2013; 119:332–338.22. Hecht JL, Konstantinopoulos PA, Awtrey CS, Soslow RA. Immunohistochemical loss of BRCA1 protein in uterine serous carcinoma. Int J Gynecol Pathol. 2014; 33:282–287.23. Evans DG, Ingham SL, Baildam A, Ross GL, Lalloo F, Buchan I, et al. Contralateral mastectomy improves survival in women with BRCA1/2-associated breast cancer. Breast Cancer Res Treat. 2013; 140:135–142.24. Drew Y, Mulligan EA, Vong WT, Thomas HD, Kahn S, Kyle S, et al. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2. J Natl Cancer Inst. 2011; 103:334–346.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Malignant Mixed Mullerian Tumor of Uterus after Tamoxifen Therapy for Breast Cancer
- One Case of Malignant Mixed Mullerian Tumor Developed in a Postmenopausal Woman under Hormone Therapy
- A Case of Primary Carcinosarcoma of the Uterine Corpus
- A Case of Malignant Mixed Mullerian Tumor of Uterus Developed After Pelvic Irradiation
- CT and MRI findings of vixed mullerian tumor: report of three cases