J Korean Ophthalmol Soc.
2011 Nov;52(11):1337-1343.
Autophagy of Human Tenon's Capsule Fibroblasts Induced by Mitomycin-C
- Affiliations
-
- 1Department of Audiology, Nambu University, Gwangju, Korea.
- 2VestibuloCochlear Research Center & Department of Microbiology, Wonkwang University, Iksan, Korea.
- 3Department of Ophthalmology, Chonbuk National University Medical School, Jeonju, Korea. ldw@jbnu.ac.kr
Abstract
- PURPOSE
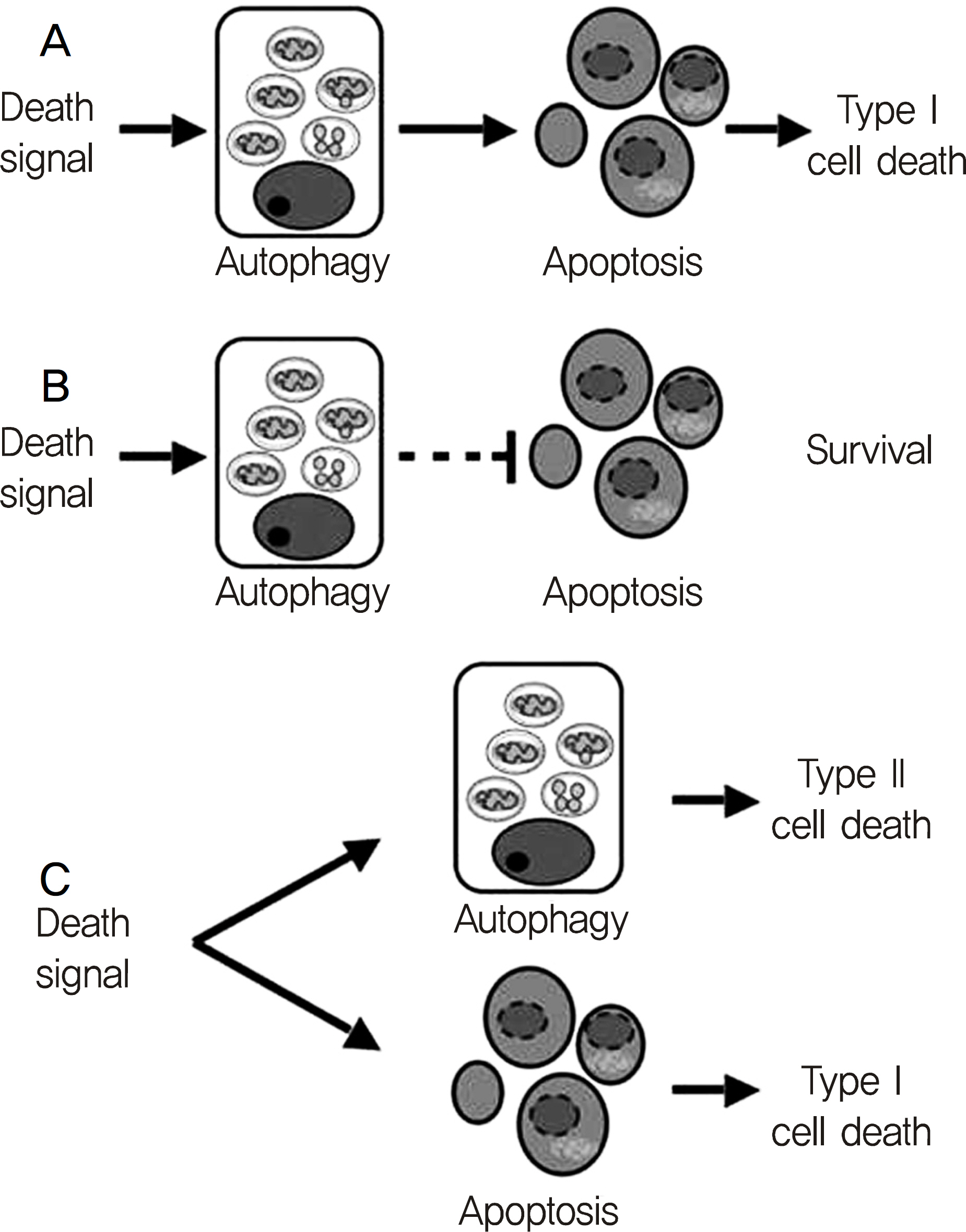
The present study investigated whether an autophagic process is involved in the apoptotic death of human tenon's capsule fibroblasts (HTCFs) caused by mitomycin-C.
METHODS
An autophagic phenotype was tested using fluorescence microscopy and flow cytometry with specific biological staining dyes including monodansylcadaverine and acridine orange and microtubule-associated protein 1 light chain 3 (LC3).
RESULTS
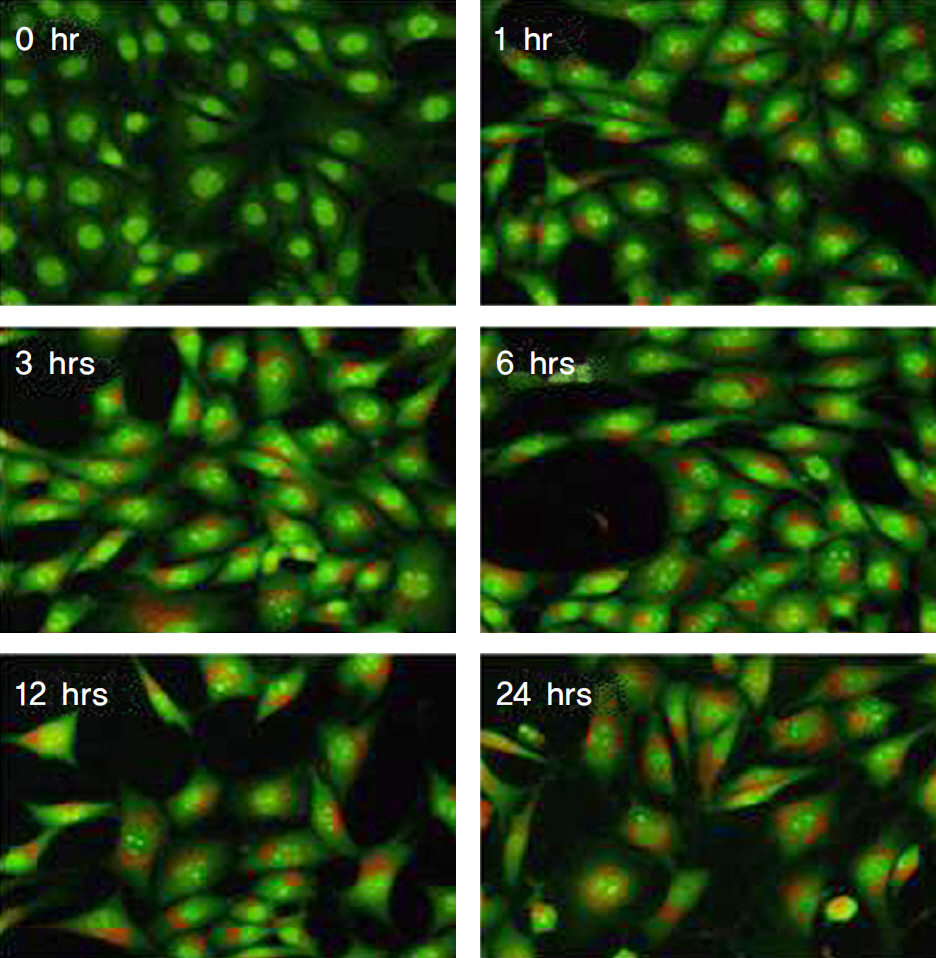
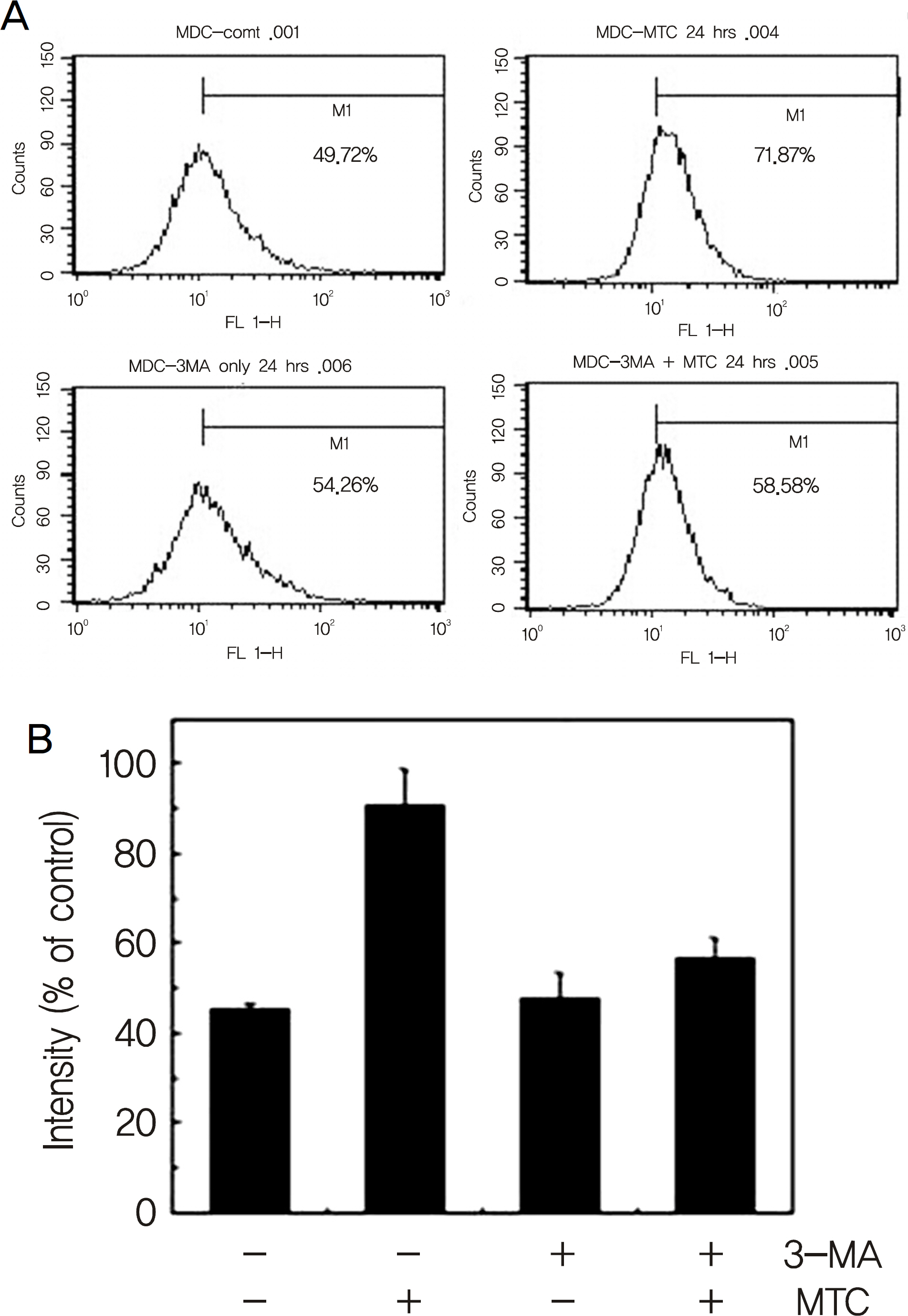
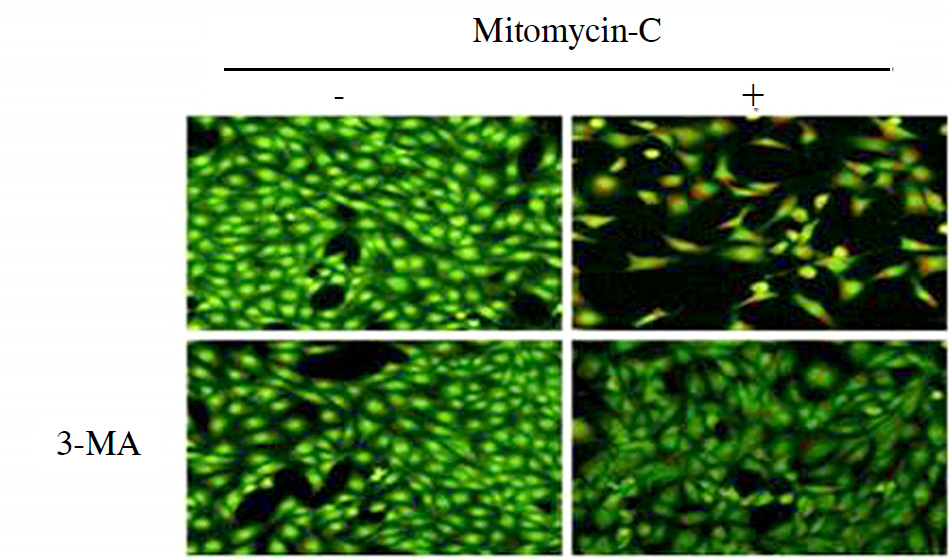
Treatment with mitomycin-C (0.4 mg/ml) increased the acidic vesicular organelles of tenon's capsule fibroblasts in a time dependent manner. Mitomycin-C induced both LC3-II cleavage and beclin-1 expression. 3-MA, a pharmacological inhibitor of autophagy, inhibited the mitomycin-C induced increase of acidic vesicular organelleS.
CONCLUSIONS
Autophagy was induced with 0.4 mg/ml mitomycin-C in tenon's capsule fibroblasts. And, autophagic mechanisms may be involved in the early stage of apoptosis of fibroblasts.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006; 443:796–802.
Article2. Levine B, Klionsky DJ. Development by self-digestion molecular mechanisms and biological functions of autophagy. Dev Cell. 2004; 6:463–77.3. Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004; 306:990–5.
Article4. Edinger AL, Thompson CB. Death by design: apoptosis, necrosis, and autophagy. Curr Opin Cell Biol. 2004; 16:663–9.
Article5. Beckers HJ, Kinders KC, Webers CA. Five-year results of trabeculectomy with mitomycin C. Graefes Arch Clin Exp Ophthalmol. 2003; 241:106–10.
Article6. Oh JH, Kim HK. The Effect of Preoperative Subconjunctival Injection of Mitomycin C and Triamcinolone in Recurrent Pterygium. J Korean Ophthalmol Soc. 2009; 50:1005–14.
Article7. Seong GJ, Park C, Kim CY, et al. Mitomycin-C induces the apoptosis of human tenon's capsule fibroblast by activation of c-Jun N-terminal kinase 1 and caspase-3 protease. Invest Ophthalmol Vis Sci. 2005; 46:3545–52.8. Bergstrom TJ, Wilkinson WS, Skuta GL, et al. The effects of subconjunctival mitomycin-C on glaucoma filtration surgery in rabbits. Arch Ophthalmol. 1991; 109:1725–30.
Article9. Choi JW, Kim TI, Tchah HW. Apoptosis of keratocytes induced by mitomycin C. J Korean Ophthalmol Soc. 2004; 45:490–9.10. Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004; 304:1500–2.11. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor sup-pressor mechanism. Oncogene. 2004; 23:2891–906.
Article12. Meijer A. Codogno JP. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004; 36:2445–62.13. Mehmet H. Caspases find a new place to hide. Nature. 2000; 403:29–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Effects Between Bevacizumab and Mitomycin C on the Survival of Fibroblasts
- Effects of Valproic Acid on the Survival of Human Tennon's Capsule Fibroblasts
- Mitomycin C-induced apoptosis in cultured human Tenon's capsule fibroblasts
- Effect of Gelrite on the Proliferation of Cultured Human Tenon's Capsule Fibroblasts
- Effect of Trusopt and Xalatan on the Proliferation of Cultured Subconjunctival Fibro blasts