J Korean Soc Spine Surg.
2011 Sep;18(3):153-162.
Analysis of Intraoperative Neurological Complications in High-Risk Spinal Surgery with the Use of Motor Evoked Potential Monitoring
- Affiliations
-
- 1Seoul Spine Institute, Sanggye Paik Hospital, College of Medicine, Inje University, Seoul, Korea. scd25@paik.ac.kr
Abstract
- STUDY DESIGN: This is retrospective study.
OBJECTIVES
To evaluate the risk of operative techniques using Motor Evoked Potential (MEP) in high-risk spinal surgery. SUMMARY OF LITERATURE REVIEW: There are few studies regarding the evaluation of operative techniques by MEP.
MATERIALS AND METHODS
We studied 33 cases that had MEP during surgery from July 2007 to March 2009. Diagnoses included post-traumatic kyphosis (PTK) in eight cases, congenital deformity in eight cases, degenerative lumbar deformity in eight cases, ankylosing spondylitis (AS) in three cases, spinal tumor in three cases, adjacent segmental disease in two cases, and post-surgical kyphosis in one case. Posterior vertebral column resection (PVCR) and pedicle subtraction osteotomy (PSO) were performed in 27 cases (81.8%) and, in the others, posterior decompression with discectomy was performed. We analyzed the risk of operative techniques and evaluated the MEP.
RESULTS
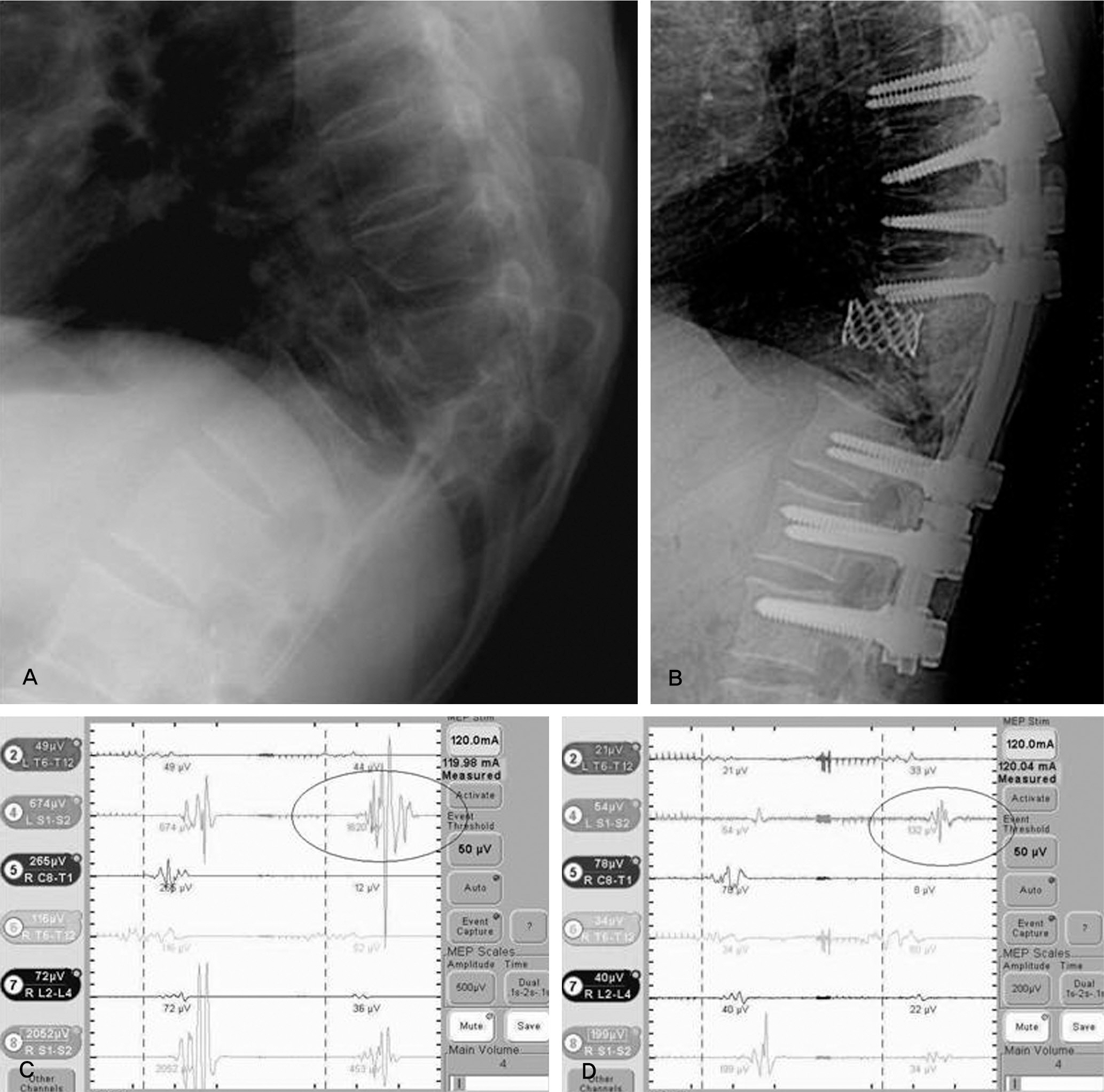
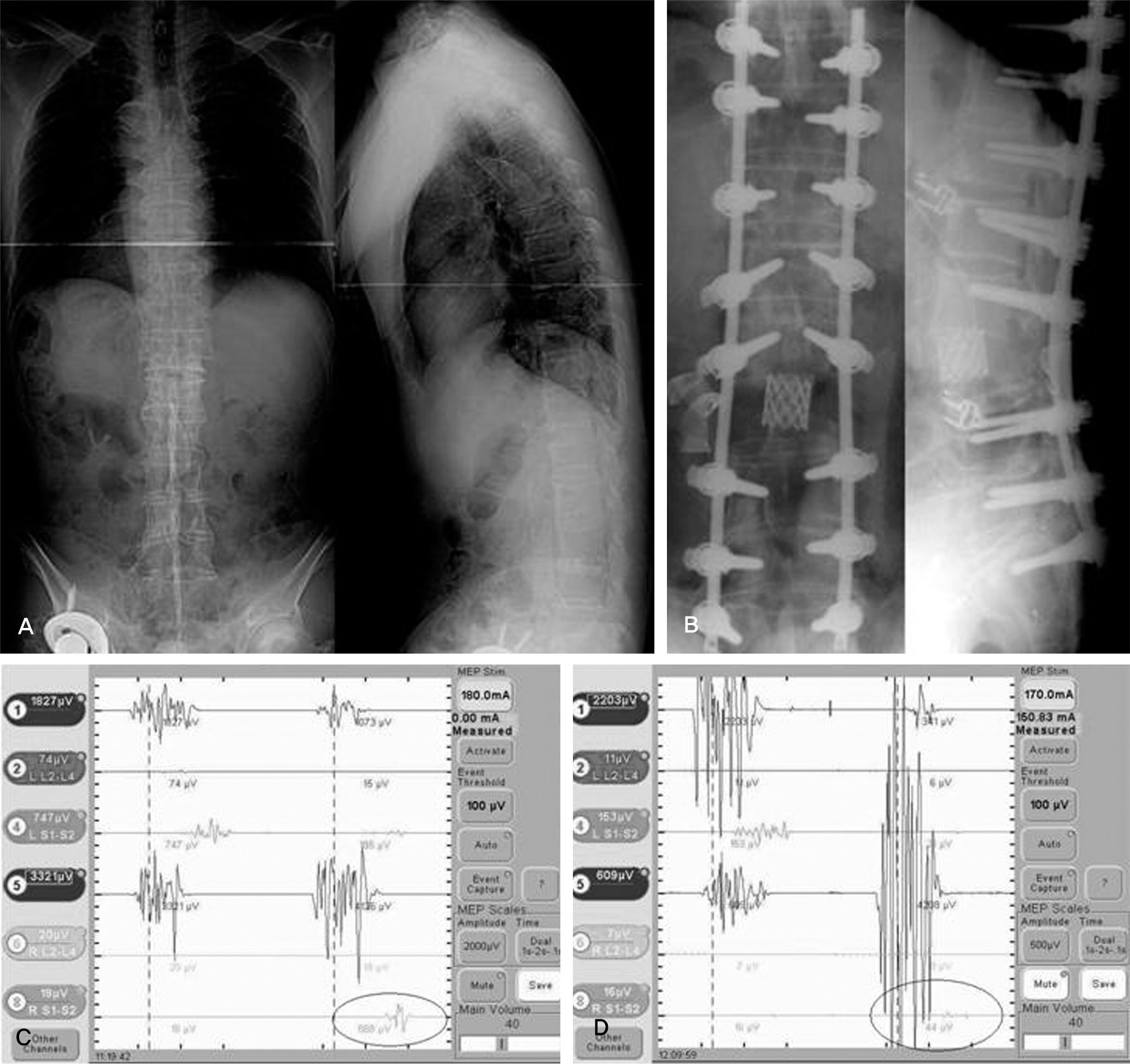
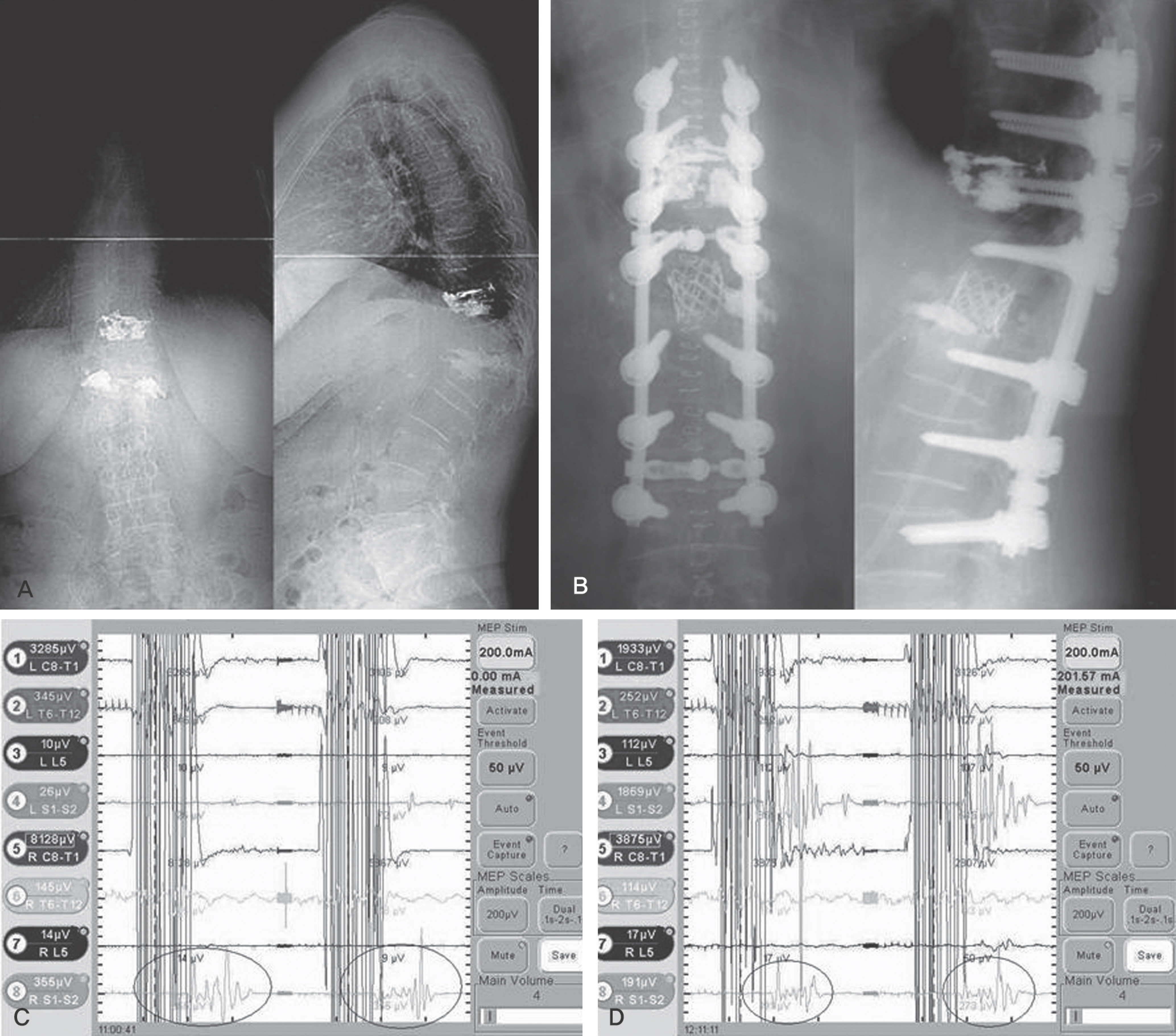
MEP showed abnormal signal change in five cases (PVCR: one case, compression and distraction: four cases). The AS case did not demonstrate neurological change after surgery. Though the PTK on T12 operated by PVCR case did not show an abnormal MEP result, neurological change was observed postoperatively. The sensitivity, specificity, percent of false negatives, and percent of false positives of MEP were 80.0%, 96.4%, 20.0%, and 3.6%, respectively.
CONCLUSIONS
MEP monitoring is a useful method to detect neurological injury during high-risk spinal surgery with satisfactory specificity. For low sensitivity and a high false negative rate, increased monitoring of cases and continuous follow-up is needed. In conclusion, compression and distraction and PVCR are high-risk techniques in kyphotic deformity correction.
Keyword
MeSH Terms
Figure
Reference
-
1. Bridwell KH, Lenke LG, Baldus C, Blanke K. Major intraoperative neurologic deficits in pediatric and adult spinal deformity patients. Incidence and etiology at one institution. Spine (Phila Pa 1976). 1998; 23:324–31.2. Nuwer MR. Spinal cord monitoring with somatosensory techniques. J Clin Neurophysiol. 1998; 15:183–93.
Article3. Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: surgical morbidity and longterm followup evaluation in 164 children and young adults. J Neurosurg. 2000; 93:183–93.
Article4. Cristante L, Herrmann HD. Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery. 1994; 35:69–74.5. Shin BJ, Lee JC, Ryu KH, Jung HW, Kim KJ, Kim YI. Intraoperative Spinal Nerve Root Injuries during Surgery for Degenerative Low Back Disease. J Korean Soc Spine Surg. 2002; 9:142–7.
Article6. Ahn DK, Choi DJ, Lee S, Jeon YW, Yang SJ. The Result of Early Decompression of Progressive Neurologic Deficit after Spine Surgery: A Case Report. J Korean Soc Spine Surg. 2007; 14:201–6.
Article7. Pechstein U, Nadstawek J, Zentner J, Schramm J. Isoflurane plus nitrous oxide versus propofol for recording of motor evoked potentials after high frequency repetitive electrical stimulation. Electroencephalogr Clin Neurophysiol. 1998; 108:175–81.
Article8. Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997; 86:10–23.9. Langeloo DD, Lelivelt A, Louis Journé e H, Slappendel R, de Kleuver M. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine (Phila Pa 1976). 2003; 28:1043–50.10. Lesser RP, Raudzens P, Lü ders H, et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986; 19:22–5.
Article11. Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995; 96:6–11.
Article12. Cioni B, Meglio M, Rossi GF. Intraoperative motor evoked potentials monitoring in spinal neurosurgery. Arch Ital Biol. 1999; 137:115–26.13. Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA. Motor evoked potential monitoring during spinal surgery: responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol. 1996; 100:375–83.
Article14. Oro J, Haghighi SS. Effects of altering core body temperature on somatosensory and motor evoked potentials in rats. Spine (Phila Pa 1976). 1992; 17:498–503.
Article15. Quinones-Hinojosa A, Lyon R, Zada G, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005; 56:982–93.16. Stoltze D, Harms J, Boyaci B. Correction of post-traumatic and congenital kyphosis: indications, techniques, results. Orthopade. 2008; 37:321–38.17. Gonzalez AA, Jeyanandarajan D, Hansen C, Zada G, Hsieh PC. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009; 27:E6.
Article18. Lieberman JA, Lyon R, Feiner J, Hu SS, Berven SH. The efficacy of motor evoked potentials in fixed sagittal imbalance deformity correction surgery. Spine (Phila Pa 1976). 2008; 33:E414–24.
Article19. Sutter MA, Eggspuehler A, Grob D, Porchet F, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring (MIOM) during 409 lumbosacral surgical procedures in 409 patients. Eur Spine J. 2007; 16(2 Suppl):S221–8.
Article20. Mummaneni PV, Dhall SS, Ondra SL, Mummaneni VP, Berven S. Pedicle subtraction osteotomy. Neurosurgery. 2008; 63(3 Suppl):S171–6.
Article21. Cheh G, Lenke LG, Padberg AM, et al. Loss of spinal cord monitoring signals in children during thoracic kyphosis correction with spinal osteotomy: why does it occur and what should you do? Spine (Phila Pa 1976). 2008; 33:1093–9.22. Kamerlink JR, Errico T, Xavier S, et al. Major intraoperative neurologic monitoring deficits in consecutive pediatric and adult spinal deformity patients at one institution. Spine (Phila Pa 1976). 2010; 35:240–5.
Article23. Suk SI, Chung ER, Lee SM, Lee JH, Kim SS, Kim JH. Posterior vertebral column resection in fixed lumbosacral deformity. Spine (Phila Pa 1976). 2005; 30:E703–10.
Article24. Suk SI, Chung ER, Kim JH, Kim SS, Lee JS, Choi WK. Posterior vertebral column resection for severe rigid scoliosis. Spine (Phila Pa 1976). 2005; 30:1682–7.
Article25. Gill JB, Levin A, Burd T, Longley M. Corrective osteotomies in spine surgery. J Bone Joint Surg Am. 2008; 90:2509–20.
Article26. Suk SI, Kim WJ, Lee CS, et al. Indications of proximal thoracic curve fusion in thoracic adolescent idiopathic scoliosis: recognition and treatment of double thoracic curve pattern in adolescent idiopathic scoliosis treated with segmental instrumentation. Spine (Phila Pa 1976). 2000; 25:2342–9.27. Lonstein JE. Cord compression. Bradford DS, editor. Moe's textbook of scoliosis and other spinal deformities. 3rd ed.Philadelphia: WB Saunders Co:;1995. 534-40.28. Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998; 88:457–70.
Article29. Modi HN, Suh SW, Yang JH, Yoon JY. False-negative transcranial motor-evoked potentials during scoliosis surgery causing paralysis: a case report with literature review. Spine (Phila Pa 1976). 2009; 34:E896–900.30. Chen X, Sterio D, Ming X, et al. Success rate of motor evoked potentials for intraoperative neurophysiologic monitoring: effects of age, lesion location, and preoperative neurologic deficits. J Clin Neurophysiol. 2007; 24:281–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intraoperative Monitoring for Tethered Cord Syndrome Using Somatosensory Evoked Potential and Motor Evoked Potential: Report of three cases
- Intraoperative Monitoring Using Somatosensory Evoked Potential during Spinal Deformity Surgery
- Monitoring of Motor and Somatosensory Evoked Potentials During Spine Surgery: Intraoperative Changes and Postoperative Outcomes
- Intraoperative Neurophysiology Monitoring for Spinal Dysraphism
- Intraoperative Neurophysiological Monitoring for Spinal Cord Tumor Surgery: Comparison of Motor and Somatosensory Evoked Potentials According to Tumor Types