World J Mens Health.
2014 Apr;32(1):28-35.
The Efficacy and Safety of Tadalafil 5 mg Once Daily in Korean Men with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia: An Integrated Analysis
- Affiliations
-
- 1Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Urology, Seoul National University Hospital, Seoul, Korea.
- 3Department of Urology, Pusan National University Hospital, Medical Research Institute, Pusan National University School of Medicine, Busan, Korea.
- 4Eli Lilly Korea, Seoul, Korea. won_ji_eon@lilly.com
- 5Eli Lilly Japan K.K., Kobe, Japan.
- 6Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, USA.
Abstract
- PURPOSE
This post hoc integrated analysis assessed the efficacy and safety of tadalafil 5 mg once daily in a large Korean population with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (BPH-LUTS).
MATERIALS AND METHODS
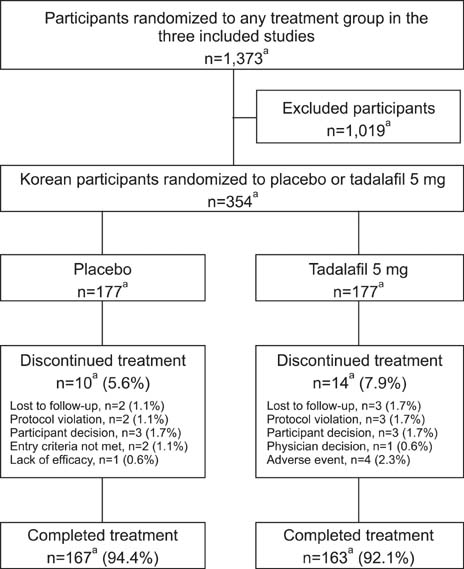
Individual Korean participant data were integrated from three 12-week, randomized, double-blind, placebo-controlled studies in Asian men with BPH-LUTS, wherein 177 Korean men received placebo and 177 received tadalafil 5 mg once daily. The primary objective was to compare the change from baseline to week 12 in total International Prostate Symptom Score (IPSS) after treatment with tadalafil versus placebo.
RESULTS
A significantly greater improvement (p<0.001) in total IPSS from baseline to week 12 was observed for tadalafil compared to placebo (least squares mean: tadalafil=-5.97; placebo=-3.94 ). Total IPSS at weeks 4 and 12, IPSS voiding and storage subscores at weeks 4, 8, and 12, and IPSS quality of life index at weeks 8 and 12 were also significantly improved (p<0.05) for tadalafil compared to placebo. There was significant improvement (p<0.001) in the patient global Impression of improvement responses and numerical improvement in the clinician global impression of improvement responses with tadalafil compared to placebo. There were no significant treatment differences for peak urine flow rate or postvoid residual volume . Few participants had treatment-emergent adverse events and there were no unexpected safety findings.
CONCLUSIONS
This integrated analysis of three randomized, placebo-controlled Asian studies confirmed tadalafil 5 mg once daily as an efficacious and well-tolerated treatment for Korean men with BPH-LUTS.
Keyword
MeSH Terms
Figure
Reference
-
1. Li MK, Garcia LA, Rosen R. Lower urinary tract symptoms and male sexual dysfunction in Asia: a survey of ageing men from five Asian countries. BJU Int. 2005; 96:1339–1354.
Article2. Homma Y, Araki I, Igawa Y, Ozono S, Gotoh M, Yamanishi T, et al. Japanese Society of Neurogenic Bladder. Clinical guideline for male lower urinary tract symptoms. Int J Urol. 2009; 16:775–790.
Article3. Lee E, Yoo KY, Kim Y, Shin Y, Lee C. Prevalence of lower urinary tract symptoms in Korean men in a community-based study. Eur Urol. 1998; 33:17–21.
Article4. Roehrborn CG. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med Clin North Am. 2011; 95:87–100.
Article5. The Korean Prostate Society. Guideline on the management of benign prostate hyperplasia [Internet]. Seoul: The Korean Prostate Society;2010. 08. 30. cited 2013 Oct 21. Available from: http://www.theprostate.org/tmp/101126_bnr1.pdf.6. Seftel AD, de la Rosette J, Birt J, Porter V, Zarotsky V, Viktrup L. Coexisting lower urinary tract symptoms and erectile dysfunction: a systematic review of epidemiological data. Int J Clin Pract. 2013; 67:32–45.
Article7. Tsukamoto T, Kumamoto Y, Masumori N, Miyake H, Rhodes T, Girman CJ, et al. Prevalence of prostatism in Japanese men in a community-based study with comparison to a similar American study. J Urol. 1995; 154:391–395.
Article8. Oh CY, Lee SH, Yoo SJ, Chung BH. Korean urologist's view of practice patterns in diagnosis and management of benign prostatic hyperplasia: a nationwide survey. Yonsei Med J. 2010; 51:248–252.
Article9. Takeda M, Nishizawa O, Imaoka T, Morisaki Y, Viktrup L. Tadalafil for the treatment of lower urinary tract symptoms in Japanese men with benign prostatic hyperplasia: results from a 12-week placebo-controlled dose-finding study with a 42-week open-label extension. LUTS. 2012; 4:110–119.
Article10. Yokoyama O, Yoshida M, Kim SC, Wang CJ, Imaoka T, Morisaki Y, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013; 20:193–201.
Article11. Takeda M, Yokoyama O, Lee SW, Murakami M, Morisaki Y, Viktrup L. Tadalafil 5 mg once-daily therapy for men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Results from a randomized, double-blind, placebo-controlled trial carried out in Japan and Korea. Int J Urol. 2014; [Epub ahead of print].12. McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007; 177:1401–1407.
Article13. Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008; 180:1228–1234.
Article14. Porst H, Kim ED, Casabé AR, Mirone V, Secrest RJ, Xu L, et al. LVHJ study team. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. 2011; 60:1105–1113.
Article15. Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012; 61:917–925.
Article16. Kim SC, Park JK, Kim SW, Lee SW, Ahn TY, Kim JJ, et al. Tadalafil administered once daily for treatment of lower urinary tract symptoms in Korean men with benign prostatic hyperplasia: results from a placebo-controlled pilot study using tamsulosin as an active control. LUTS. 2011; 3:86–93.
Article17. Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The Measurement Committee of the American Urological Association. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992; 148:1549–1557.
Article18. Viktrup L, Hayes RP, Wang P, Shen W. Construct validation of patient global impression of severity (PGI-S) and improvement (PGI-I) questionnaires in the treatment of men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. BMC Urol. 2012; 12:30.
Article19. Roehrborn CG, Kaminetsky JC, Auerbach SM, Montelongo RM, Elion-Mboussa A, Viktrup L. Changes in peak urinary flow and voiding efficiency in men with signs and symptoms of benign prostatic hyperplasia during once daily tadalafil treatment. BJU Int. 2010; 105:502–507.
Article20. Kawabe K, Yoshida M, Homma Y. Silodosin Clinical Study Group. Silodosin, a new alpha1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006; 98:1019–1024.21. Roehrborn CG, Chapple C, Oelke M, Cox D, Esler A, Viktrup L. Effects of tadalafil once daily on maximum urinary flow rate in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. J Urol. 2014; 191:1045–1050.
Article22. American Urological Association. American Urologial Association Guideline: Management of Benign Hyperplasia (BPH). Linthicum (MD): American Urological Association;2010. cited 2013 Nov 18. Available from: http://www.auanet.org/common/pdf/education/clinical-guidance/Benign-Prostatic-Hyperplasia.pdf.23. Giuliano F, Ückert S, Maggi M, Birder L, Kissel J, Viktrup L. The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol. 2013; 63:506–516.
Article24. Eli Lilly and Company. U.S. Food and Drug Administration prescribing information [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;2011. 10. cited 2014 Feb 16. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021368s20s21lbl.pdf.25. Donatucci CF, Brock GB, Goldfischer ER, Pommerville PJ, Elion-Mboussa A, Kissel JD, et al. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a 1-year, open-label extension study. BJU Int. 2011; 107:1110–1116.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety and Effectiveness of Once-Daily Tadalafil (5 mg) Therapy in Korean Men with Benign Prostatic Hyperplasia/Lower Urinary Tract Symptoms in a Real-World Clinical Setting: Results from a Post-Marketing Surveillance Study
- Urinary Tract Symptoms (LUTS) Secondary to Benign Prostatic Hyperplasia (BPH) and LUTS/BPH with Erectile Dysfunction in Asian Men: A Systematic Review Focusing on Tadalafil
- Efficacy and Safety of Alfuzosin 10 mg Once Daily in Patients with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia: A 6-Months Study in Real Life Practice
- Open-label, Intermittent Dose, Prospective Study Evaluating the Effects of Tadalafil on Lower Urinary Tract Symptoms and Erectile Function in Patients with Benign Prostatic Hyperplasia: Continuation and Durability of Effects
- Benign Prostatic Hyperplasia and Sexual Dysfunction