Nutr Res Pract.
2013 Jun;7(3):160-165.
Peanut sprout ethanol extract inhibits the adipocyte proliferation, differentiation, and matrix metalloproteinases activities in mouse fibroblast 3T3-L1 preadipocytes
- Affiliations
-
- 1Department of Food Science and Nutrition, Dankook University, 126 Jukjeon-dong, Suji-gu, Yongin-si, Gyeonggi 448-701, Korea. aewhaha@dankook.ac.kr
- 2Department of Food and Nutrition, Eulji University, Seongnam, Gyeonggi 461-723, Korea.
- 3Department of Food Engineering, Dankook University, Cheonan, Chungnam 330-714, Korea.
Abstract
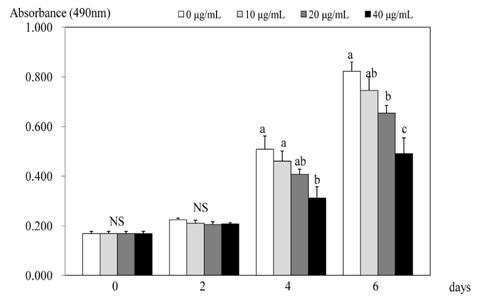
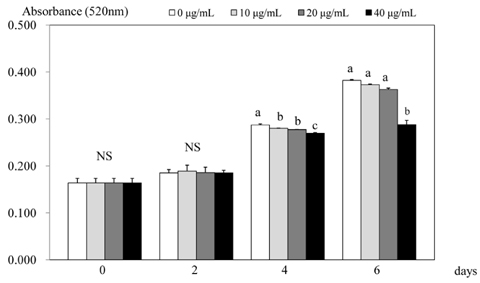
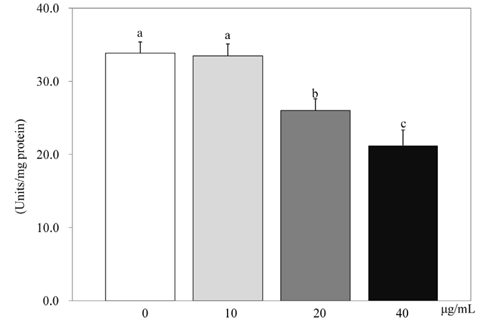
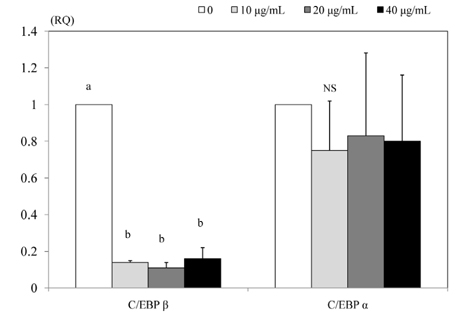
- 3T3-L1 preadipocyte were differentiated to adipocytes, and then treated with 0, 10, 20, and 40 microg/mL of peanut sprout ethanol extract (PSEE). The main component of PSEE is resveratrol which contained 5.55 mg/mL of resveratrol. The MTT assay, Oil-Red O staining, glycerol-3-phosphate dehydrogenase (GPDH) activity, and the triglyceride concentration were determined in 3T3-L1 cells. MMP-2 and MMP-9 activities as well as mRNA expressions of C/EBP beta and C/EBP alpha were also investigated. As the concentration of PSEE in adipocytes increased, the cell proliferation was decreased in a dose-dependent manner from 4 days of incubation (P < 0.05). The GDPH activity (P < 0.05) and the triglyceride concentration (P < 0.05) were decreased as the PSEE treatment concentration increased. The mRNA expression of C/EBPbeta in 3T3-L1 cells was significantly low in groups of PSEE-treated, compared with control group (P < 0.05). The MMP-9 (P < 0.05) and MMP-2 (P < 0.05) activities were decreased in a dose-dependent manner as the PSEE concentration increased from 20 microg/mL. In conclusion, it was found that PSEE has an effect on restricting proliferation and differentiation of adipocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. 1994; 14:99–129.
Article2. Camp HS, Ren D, Leff T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol Med. 2002; 8:442–447.
Article3. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000; 14:1293–1307.
Article4. Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001; 50:2080–2086.5. Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci. 2002; 957:210–229.
Article6. de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007; 35:1156–1160.
Article7. Das S, Alagappan VK, Bagchi D, Sharma HS, Maulik N, Das DK. Coordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol consumption: a potential mechanism for resveratrol preconditioning of the heart. Vascul Pharmacol. 2005; 42:281–289.8. Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox'? Eur J Endocrinol. 1998; 138:619–620.
Article9. Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002; 30:1064–1070.
Article10. Szkudelska K, Nogowski L, Szkudelski T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol. 2009; 113:17–24.
Article11. Dal-Pan A, Blanc S, Aujard F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol. 2010; 10:11.
Article12. Gu X, Creasy L, Kester A, Zeece M. Capillary electrophoretic determination of resveratrol in wines. J Agric Food Chem. 1999; 47:3223–3227.
Article13. Wang KH, Lai YH, Chang JC, Ko TF, Shyu SL, Chiou RY. Germination of peanut kernels to enhance resveratrol biosynthesis and prepare sprouts as a functional vegetable. J Agric Food Chem. 2005; 53:242–246.
Article14. Kang HI, Kim JY, Park KW, Kang JS, Choi MR, Moon KD, Seo KI. Resveratrol content and nutritional components in peanut sprouts. Korean J Food Preserv. 2010; 17:384–390.15. Kang HI, Kim JY, Kwon SJ, Park KW, Kang JS, Seo KI. Antioxidative effects of peanut sprout extracts. J Korean Soc Food Sci Nutr. 2010; 39:941–946.
Article16. Lin BS, Lien TF, Chao MR, Lai TY, Chang JC, Chou SJ, Liao HF, Chiou RY. Toxicological and nutraceutical assessments of peanut sprouts as daily supplements to feed Sprague-Dawley rats for 18 weeks. J Sci Food Agric. 2008; 88:2201–2207.
Article17. Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975; 5:19–27.
Article18. Lee MS, Kim CT, Kim CJ, Cho YJ, Kim Y. Effects of Portulaca oleracea L. extract on lipolysis and hormone sensitive lipase (HSL) gene expression in 3T3-L1 adipocytes. Korean J Nutr. 2006; 39:742–747.19. Kim MJ, Kim Y, Chung JH, Kim JW, Kim HK. The effect of caffeine on 3T3-L1 adipocyte differentiation: a nutrigenomical approach. Korean J Nutr. 2005; 38:649–655.20. Chon JW, Sung JH, Hwang EJ, Park YK. Chlorella methanol extract reduces lipid accumulation in and increases the number of apoptotic 3T3-L1 cells. Ann N Y Acad Sci. 2009; 1171:183–189.
Article21. Tomiyama K, Nakata H, Sasa H, Arimura S, Nishio E, Watanabe Y. Wortmannin, a specific phosphatidylinositol 3-kinase inhibitor, inhibits adipocytic differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 1995; 212:263–269.
Article22. Ramírez-Zacarías JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992; 97:493–497.
Article23. Na MH, Seo EY, Kim WK. Effects of α-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells. Nutr Res Pract. 2009; 3:265–271.
Article24. Kwon SY, Kang KJ. The effect of conjugated linoleic acid isomers on the cell proliferation, apoptosis and expressions of uncoupling protein (UCP) genes during differentiation of 3T3-L1 preadipocytes. Korean J Nutr. 2004; 37:533–539.25. Kyoya T, Ishida A, Nakashima K, Nakajima I, Toyoda A, Nakamura Y, Katsumata M. The effects of concentrations of lysine in media on differentiation of 3T3-L1 preadipocytes. Anim Sci J. 2011; 82:565–570.
Article26. Olofsson LE, Orho-Melander M, William-Olsson L, Sjöholm K, Sjöström L, Groop L, Carlsson B, Carlsson LM, Olsson B. CCAAT/enhancer binding protein alpha (C/EBPalpha) in adipose tissue regulates genes in lipid and glucose metabolism and a genetic variation in C/EBPalpha is associated with serum levels of triglycerides. J Clin Endocrinol Metab. 2008; 93:4880–4886.
Article27. Cho KJ, Moon HE, Moini H, Packer L, Yoon DY, Chung AS. Alpha-lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways. J Biol Chem. 2003; 278:34823–34833.
Article28. Hong MK, Cho KY, Oh SJ, Kim KM, Yu SJ, Jung SS. Implications of the activation of matrix metalloproteinase-2 (MMP-2) on the metastasis in breast cancer. J Korean Surg Soc. 2002; 62:18–25.
Article29. Rural Development Administration, National Institute of Crop Science [Internet]. Suwon: Rural Development Administration;2010. cited 2010 October 14. Available from: http://rda.korea.kr/gonews.30. Shin HD. Anti-obesity effect of vegetable sprouts in 3T3-L1 adipocytes and rats [master's thesis]. Gwangju: Chosun University;2007.31. Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979; 254:273–275.
Article32. Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008; 22:1367–1371.
Article33. Zhang XH, Huang B, Choi SK, Seo JS. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr Res Pract. 2012; 6:286–293.
Article34. Kang NE, Ha AW, Kim JY, Kim WK. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes. Nutr Res Pract. 2012; 6:499–504.
Article35. Park HJ, Yang JY, Ambati S, Della-Fera MA, Hausman DB, Rayalam S, Baile CA. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. J Med Food. 2008; 11:773–783.
Article36. Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987; 235:442–447.
Article37. Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. 2001; 152:693–703.
Article38. Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, Song L. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006; 78:2564–2570.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of quercetin on cell differentiation and adipogenesis in 3T3-L1 adipocytes
- Effects of (6)-gingerol, ginger component on adipocyte development and differentiation in 3T3-L1
- The Effects of Epigallocatechin on Adipogenesis of 3T3-L1 Preadipocytes
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3