J Breast Cancer.
2013 Sep;16(3):300-307. 10.4048/jbc.2013.16.3.300.
Comparison of Electron and X-Ray Beams for Tumor Bed Boost Irradiation in Breast-Conserving Treatment
- Affiliations
-
- 1Department of Radiation Oncology, Kyungpook National University School of Medicine, Daegu, Korea. jckim@knu.ac.kr
- KMID: 2286381
- DOI: http://doi.org/10.4048/jbc.2013.16.3.300
Abstract
- PURPOSE
This study aimed to compare the dosimetric profiles of electron beams (EB) and X-ray beams (XB) for boosting irradiation in breast cancer patients who underwent breast-conserving surgery and postoperative radiotherapy.
METHODS
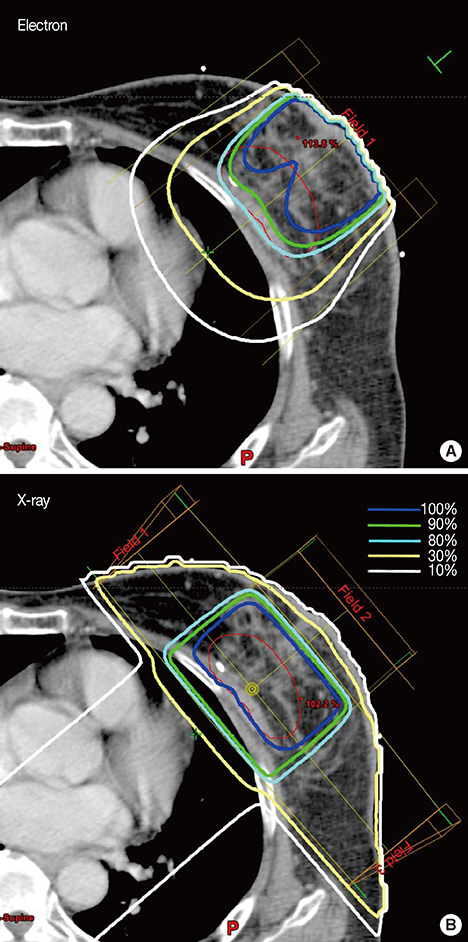
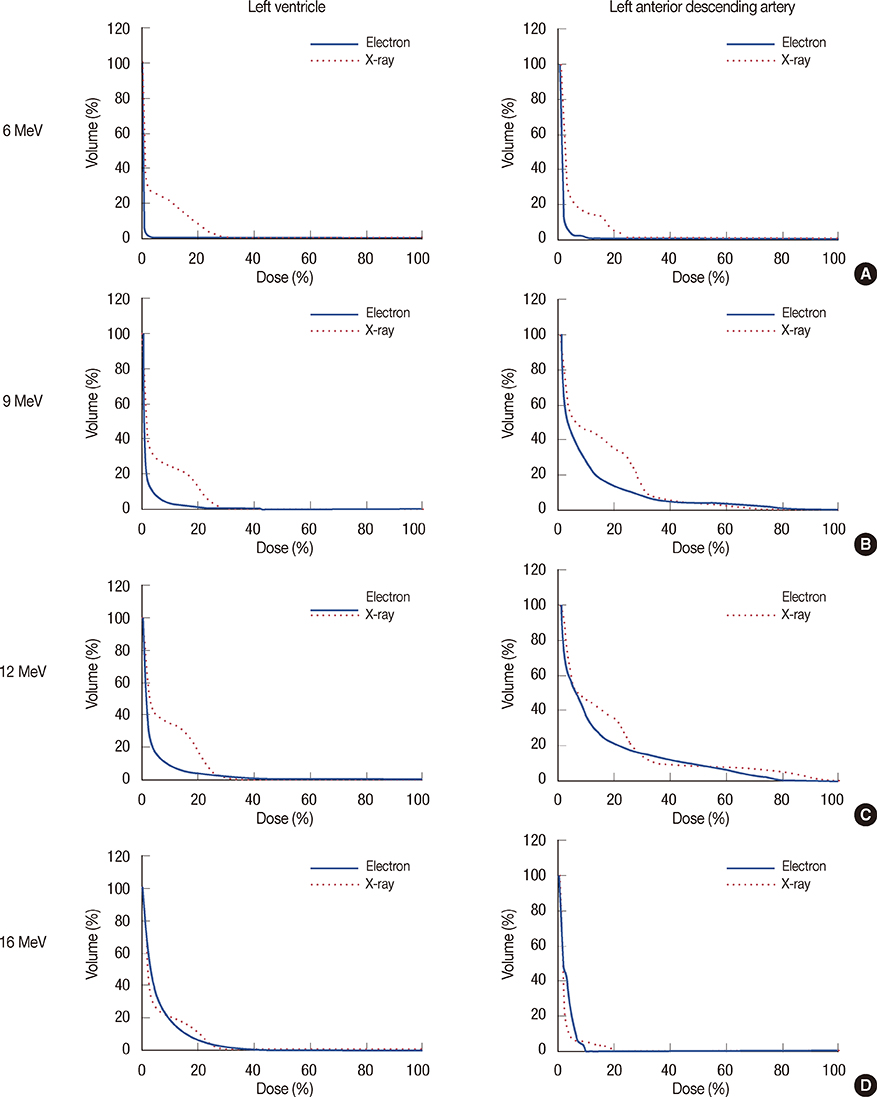
For 131 breast cancer patients who underwent breast-conserving surgery, we compared plans for EB and XB boost irradiation after whole-breast irradiation. The organs at risk (OAR) included the cardiac chambers, coronary arteries, ipsilateral lung, and skin. The conformity index (CI), inhomogeneity index (IHI), and dose-volume parameters for the planning target volume (PTV), and OAR were calculated. Postradiotherapy chest computed tomography scans were performed to detect radiation pneumonitis.
RESULTS
XB plans showed a significantly better CI and IHI for the PTVs, compared to the EB plans. Regarding OAR sparing, the XB reduced the high-dose volume at the expense of an increased low-dose volume. In 33 patients whose radiation fields included nipples, IHI was higher in the EB plans, whereas the presence of a nipple in the radiation field did not interfere with the XB. EB-treated patients developed more subclinical radiation pneumonitis.
CONCLUSION
XB plans were superior to EB plans in terms of PTV coverage (homogeneity and conformity) and high-dose volume sparing in OAR when used as boost irradiation after breast-conserving surgery. A disadvantage of the XB plan was an increased low-dose volume in the OAR, but this was offset by the increased electron energy. Consequently, tailored plans with either XB or EB are necessary to adapt to patient anatomic variance and tumor bed geometric properties.
MeSH Terms
Figure
Reference
-
1. Romestaing P, Lehingue Y, Carrie C, Coquard R, Montbarbon X, Ardiet JM, et al. Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997; 15:963–968.
Article2. Collette S, Collette L, Budiharto T, Horiot JC, Poortmans PM, Struikmans H, et al. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881-10882 'boost versus no boost'. Eur J Cancer. 2008; 44:2587–2599.
Article3. Khan FM. The Physics of Radiation Therapy. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2009. p. 264–310.4. Kim LH, DeCesare S, Vicini F, Yan D. Effect of lumpectomy cavity volume change on the clinical target volume for accelerated partial breast irradiation: a deformable registration study. Int J Radiat Oncol Biol Phys. 2010; 78:1121–1126.
Article5. Hurkmans C, Admiraal M, van der Sangen M, Dijkmans I. Significance of breast boost volume changes during radiotherapy in relation to current clinical interobserver variations. Radiother Oncol. 2009; 90:60–65.
Article6. Libshitz HI, Shuman LS. Radiation-induced pulmonary change: CT findings. J Comput Assist Tomogr. 1984; 8:15–19.7. Choi YW, Munden RF, Erasmus JJ, Park KJ, Chung WK, Jeon SC, et al. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. Radiographics. 2004; 24:985–997.
Article8. Kovacs A, Hadjiev J, Lakosi F, Glavak C, Antal G, Bogner P, et al. Comparison of photon with electron boost in treatment of early stage breast cancer. Pathol Oncol Res. 2008; 14:193–197.
Article9. Toscas JI, Linero D, Rubio I, Hidalgo A, Arnalte R, Escudé L, et al. Boosting the tumor bed from deep-seated tumors in early-stage breast cancer: a planning study between electron, photon, and proton beams. Radiother Oncol. 2010; 96:192–198.
Article10. Alexander A, Soisson E, Hijal T, Sarfehnia A, Seuntjens J. Comparison of modulated electron radiotherapy to conventional electron boost irradiation and volumetric modulated photon arc therapy for treatment of tumour bed boost in breast cancer. Radiother Oncol. 2011; 100:253–258.
Article11. Seddon B, Cook A, Gothard L, Salmon E, Latus K, Underwood SR, et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol. 2002; 64:53–63.
Article12. Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006; 24:4100–4106.
Article13. McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011; 100:167–175.
Article14. Rutqvist LE, Lax I, Fornander T, Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys. 1992; 22:887–896.
Article15. Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005; 6:557–565.
Article16. Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, Blazing M, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005; 63:214–223.
Article17. Erven K, Jurcut R, Weltens C, Giusca S, Ector J, Wildiers H, et al. Acute radiation effects on cardiac function detected by strain rate imaging in breast cancer patients. Int J Radiat Oncol Biol Phys. 2011; 79:1444–1451.
Article18. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013; 368:987–998.
Article19. Bouchardy C, Rapiti E, Usel M, Majno SB, Vlastos G, Benhamou S, et al. Excess of cardiovascular mortality among node-negative breast cancer patients irradiated for inner-quadrant tumors. Ann Oncol. 2010; 21:459–465.
Article20. Rodrigues G, Lock M, D'Souza D, Yu E, Van Dyk J. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer: a systematic review. Radiother Oncol. 2004; 71:127–138.
Article21. Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006; 66:1281–1293.
Article22. Wennberg B, Gagliardi G, Sundbom L, Svane G, Lind P. Early response of lung in breast cancer irradiation: radiologic density changes measured by CT and symptomatic radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002; 52:1196–1206.
Article23. Mah K, Keane TJ, Van Dyk J, Braban LE, Poon PY, Hao Y. Quantitative effect of combined chemotherapy and fractionated radiotherapy on the incidence of radiation-induced lung damage: a prospective clinical study. Int J Radiat Oncol Biol Phys. 1994; 28:563–574.
Article24. Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001; 345:1378–1387.
Article25. Perez CA, Taylor ME, Halverson K, Garcia D, Kuske RR, Lockett MA. Brachytherapy or electron beam boost in conservation therapy of carcinoma of the breast: a nonrandomized comparison. Int J Radiat Oncol Biol Phys. 1996; 34:995–1007.
Article26. Mansfield CM, Komarnicky LT, Schwartz GF, Rosenberg AL, Krishnan L, Jewell WR, et al. Ten-year results in 1070 patients with stages I and II breast cancer treated by conservative surgery and radiation therapy. Cancer. 1995; 75:2328–2336.
Article27. Touboul E, Belkacemi Y, Lefranc JP, Uzan S, Ozsahin M, Korbas D, et al. Early breast cancer: influence of type of boost (electrons vs iridium-192 implant) on local control and cosmesis after conservative surgery and radiation therapy. Radiother Oncol. 1995; 34:105–113.
Article28. Horton JK, Halle JS, Chang SX, Sartor CI. Comparison of three concomitant boost techniques for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2006; 64:168–175.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor bed volumetric changes during breast irradiation for the patients with breast cancer
- Evaluation of Electron Boost Fields based on Surgical Clips and Operative Scars in Definitive Breast Irradiation
- Displacement of Surgical Clips in Patients with Human AcellularDermal Matrix in the Excision Cavity during Whole Breast IrradiationFollowing Breast-Conserving Surgery
- Long-term oncological outcomes of hypofractionated versus conventional fractionated whole breast irradiation with simultaneous integrated boost in early-stage breast cancer
- Treatment Outcome and Analysis of the Prognostic Factors of Ductal Carcinoma in situ Treated with Breast Conserving Surgery and Radiotherapy