J Breast Cancer.
2015 Mar;18(1):1-7. 10.4048/jbc.2015.18.1.1.
Clinicopathological Significance of Dual-Specificity Protein Phosphatase 4 Expression in Invasive Ductal Carcinoma of the Breast
- Affiliations
-
- 1Department of Pathology, Hanyang University College of Medicine, Seoul, Korea. sspaik@hanyang.ac.kr
- 2Department of Surgery, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2286330
- DOI: http://doi.org/10.4048/jbc.2015.18.1.1
Abstract
- PURPOSE
Dual-specificity protein phosphatase 4 (DUSP4), also known as mitogen-activated protein kinase phosphatase (MKP) 2 is a member of the inducible nuclear MKP group. The role of DUSP4 in cancer development and progression appears to vary with the type of malignancy. The purpose of this study was to investigate DUSP4 expression in a case series of invasive ductal carcinoma of the breast.
METHODS
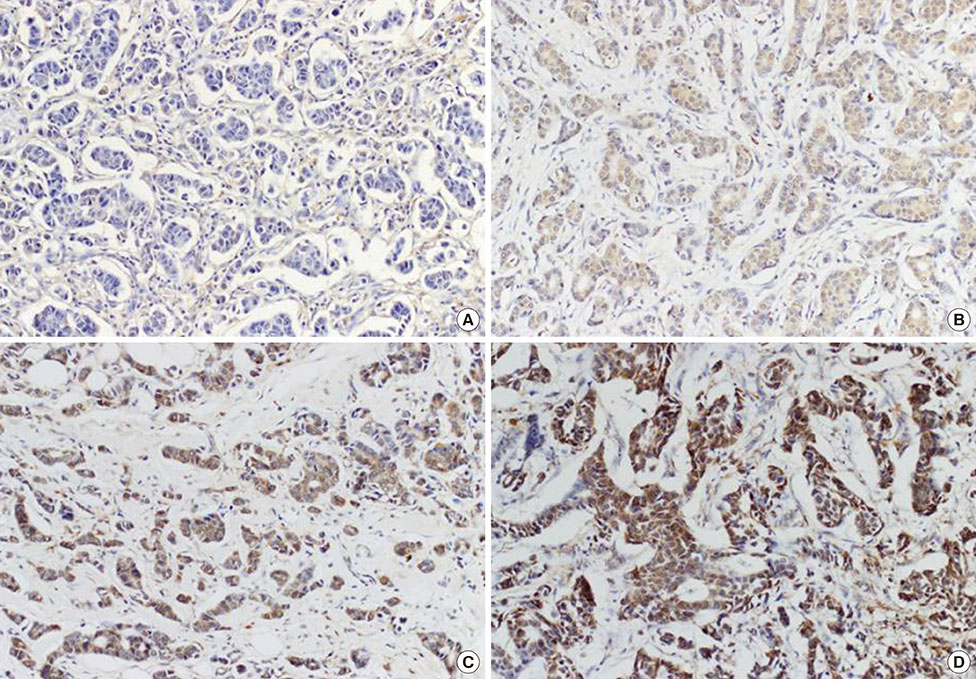
We constructed tissue microarrays consisting of 16, 14, 47, and 266 cases of normal breast tissue, usual ductal hyperplasia, ductal carcinoma in situ, and invasive ductal carcinoma, respectively. DUSP4 expression was investigated by immunohistochemistry.
RESULTS
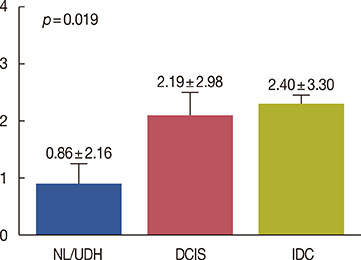
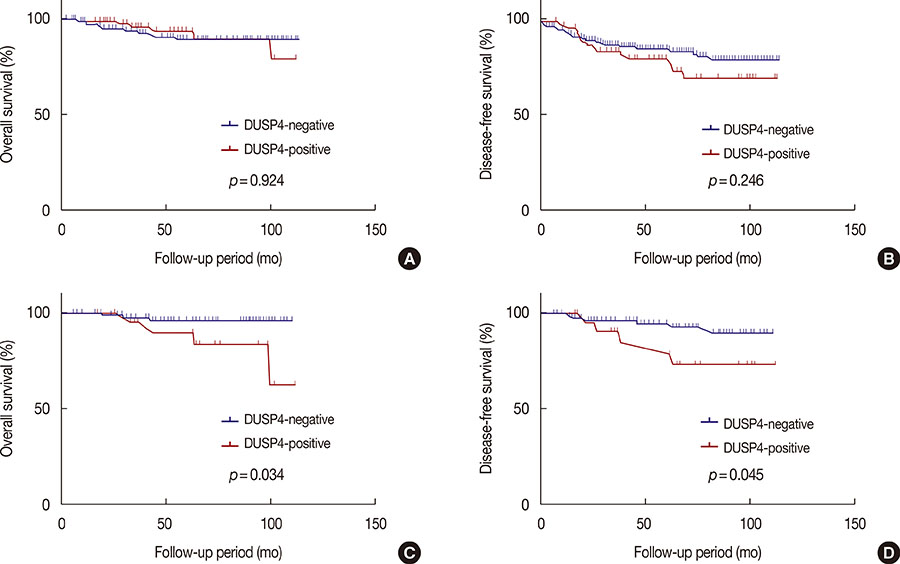
Cytoplasmic DUSP4 expression was observed. DUSP4 was more frequently expressed in malignant than in benign cases (p=0.024). The mean DUSP4 expression score was significantly higher in malignant tumors than in benign lesions (p=0.019). DUSP4 expression was significantly correlated with a larger tumor size (>2 cm, p=0.015). There was no significant correlation between overall survival or disease-free survival and DUSP4 expression in all 266 patients. We evaluated the impact of DUSP4 expression on the survival of 120 patients with T1-stage tumors. Interestingly, Kaplan-Meier survival curves revealed that DUSP4 expression had a significant effect on both overall patient survival (p=0.034, log-rank test) and disease-free survival (p=0.045, log-rank test). In early T-stage breast cancer, DUSP4 expression was associated with a worse prognosis.
CONCLUSION
DUSP4 is frequently upregulated in breast malignancy, and may play an important role in cancer development and progression. In addition, it may be a marker of adverse prognosis, especially in patients with early T1-stage cancer.
MeSH Terms
Figure
Reference
-
1. DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011; 61:409–418.
Article2. Schmidt M, Voelker HU, Kapp M, Krockenberger M, Dietl J, Kammerer U. Glycolytic phenotype in breast cancer: activation of Akt, up-regulation of GLUT1, TKTL1 and down-regulation of M2PK. J Cancer Res Clin Oncol. 2010; 136:219–225.
Article3. Malhotra GK, Zhao X, Band H, Band V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther. 2010; 10:955–960.
Article4. Jang SM, Han H, Jang KS, Jun YJ, Jang SH, Min KW, et al. The glycolytic phenotype is correlated with aggressiveness and poor prognosis in invasive ductal carcinomas. J Breast Cancer. 2012; 15:172–180.
Article5. Bertolo C, Guerrero D, Vicente F, Cordoba A, Esteller M, Ropero S, et al. Differences and molecular immunohistochemical parameters in the subtypes of infiltrating ductal breast cancer. Am J Clin Pathol. 2008; 130:414–424.
Article6. Gröschl B, Bettstetter M, Giedl C, Woenckhaus M, Edmonston T, Hofstädter F, et al. Expression of the MAP kinase phosphatase DUSP4 is associated with microsatellite instability in colorectal cancer (CRC) and causes increased cell proliferation. Int J Cancer. 2013; 132:1537–1546.
Article7. De Vriendt V, De Roock W, Di Narzo AF, Tian S, Biesmans B, Jacobs B, et al. DUSP 4 expression identifies a subset of colorectal cancer tumors that differ in MAPK activation, regardless of the genotype. Biomarkers. 2013; 18:516–524.
Article8. Saigusa S, Inoue Y, Tanaka K, Toiyama Y, Okugawa Y, Shimura T, et al. Decreased expression of DUSP4 is associated with liver and lung metastases in colorectal cancer. Med Oncol. 2013; 30:620.
Article9. Liu Y, Du F, Chen W, Yao M, Lv K, Fu P. Knockdown of dual specificity phosphatase 4 enhances the chemosensitivity of MCF-7 and MCF-7/ ADR breast cancer cells to doxorubicin. Exp Cell Res. 2013; 319:3140–3149.
Article10. Jang SM, Sim J, Han H, Ahn HI, Kim H, Yi K, et al. Clinicopathological significance of CADM4 expression in invasive ductal carcinoma of the breast. J Clin Pathol. 2013; 66:681–686.
Article11. Jang SM, Han H, Jun YJ, Jang SH, Min KW, Sim J, et al. Clinicopathological significance of CADM4 expression, and its correlation with expression of E-cadherin and Ki-67 in colorectal adenocarcinomas. J Clin Pathol. 2012; 65:902–906.
Article12. Waha A, Felsberg J, Hartmann W, von dem, Mikeska T, Joos S, et al. Epigenetic downregulation of mitogen-activated protein kinase phosphatase MKP-2 relieves its growth suppressive activity in glioma cells. Cancer Res. 2010; 70:1689–1699.
Article13. Armes JE, Hammet F, de Silva M, Ciciulla J, Ramus SJ, Soo WK, et al. Candidate tumor-suppressor genes on chromosome arm 8p in earlyonset and high-grade breast cancers. Oncogene. 2004; 23:5697–5702.
Article14. Baglia ML, Cai Q, Zheng Y, Wu J, Su Y, Ye F, et al. Dual specificity phosphatase 4 gene expression in association with triple-negative breast cancer outcome. Breast Cancer Res Treat. 2014; 148:211–220.
Article15. Wang HY, Cheng Z, Malbon CC. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 2003; 191:229–237.
Article16. Gaedcke J, Grade M, Jung K, Camps J, Jo P, Emons G, et al. Mutated KRAS results in overexpression of DUSP4, a MAP-kinase phosphatase, and SMYD3, a histone methyltransferase, in rectal carcinomas. Genes Chromosomes Cancer. 2010; 49:1024–1034.
Article17. Yip-Schneider MT, Lin A, Marshall MS. Pancreatic tumor cells with mutant K-ras suppress ERK activity by MEK-dependent induction of MAP kinase phosphatase-2. Biochem Biophys Res Commun. 2001; 280:992–997.
Article18. Teutschbein J, Haydn JM, Samans B, Krause M, Eilers M, Schartl M, et al. Gene expression analysis after receptor tyrosine kinase activation reveals new potential melanoma proteins. BMC Cancer. 2010; 10:386.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Uncoupling Protein 2 (UCP2) and p53 Expression in Invasive Ductal Carcinoma of Breast
- Expression of p21, p53 and bcl-2 Proteins in Invasive Ductal Carcinoma of the Breast
- Clinicopathological Significance of SMAD4 Expression in Breast Cancer
- Significance of Expression of p16, Cyclin D1, Rb, and p53 Protein and Correlation with Clinicopathologic Prognostic Factors in Invasive Ductal Carcinoma of the Breast
- Comparison of the Expression of Variants of CD44 between Node-positive and Node-negative Breast Carcinomas