Korean J Nutr.
2010 Feb;43(1):12-25. 10.4163/kjn.2010.43.1.12.
Bioavailability and Digestibility of Organic Calcium Sources by Bone Health Index
- Affiliations
-
- 1College of Life Sciences and Biotechnology, Korea University, Seoul 136-702, Korea.
- 2Korea Food Research Institute, Sungnam 463-746, Korea. kem@kfri.re.kr
- 3Sampoong B&F Co. Ltd. Seoul 143-960, Korea.
- KMID: 2267960
- DOI: http://doi.org/10.4163/kjn.2010.43.1.12
Abstract
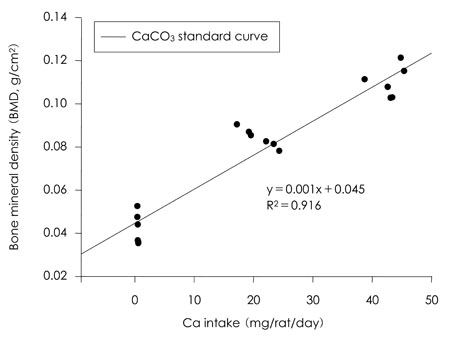
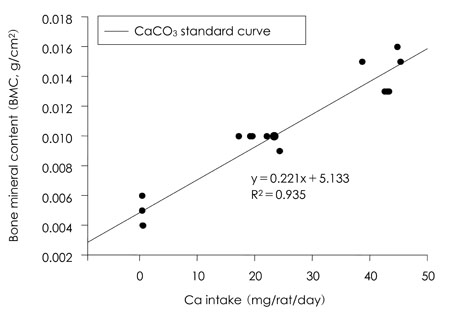
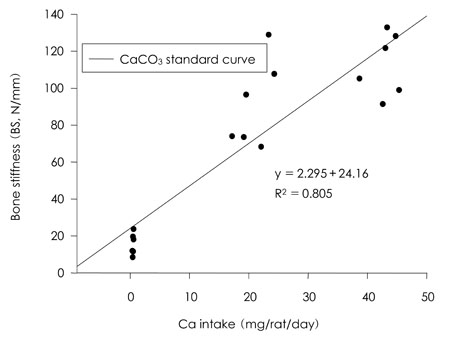
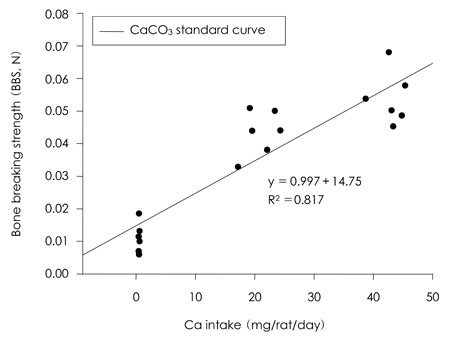
- This study was carried out to evaluate the bioavailabilities and the digestibilities of oligopeptide chelated (peptide-Ca), anchovy bone (anchovy-Ca) and methionine hydroxyl analogue (MHA-Ca) calcium compared to those of calcium carbonate in rats. In exp1, CaCO3, were added to the basal diet at level of 0, 30 and 60% calcium of the AIN-93G diet. In test groups, peptide-Ca, anchovy-Ca and MHA-Ca, were added to the basal diet to provide calcium at the level of 40% of AIN-93G. In exp1, the bioavailabilities were evaluated from the regression equation of the ratios of theological/actual calcium intakes of each dietary treatment. In exp2, urine and feces was to evaluate the true- and apparent digestibility and apparent retention. In exp1, Ca-60% group had higher bone mineral density (BMD), bone mineral content (BMC) and bone breaking strength (BBS) than those of the other standard groups. The bone weight and ash content of the peptide-Ca and anchovy-Ca groups were significantly higher than those of the MHA-Ca. Bone calcium content were not significantly different from the test group. The bioavailability of the MHA-Ca group was shown higher BMD (71%), BS (38%) and BBS (27%) compared to another control group. But the regression coefficient for BMD, BS and BBS were lower compare with that of bone ash and BMC. In exp2, the true- and apparent digestibility of test groups were shown to over 90%. Peptide-Ca was not significantly different from other test group, but digestibility and retention were higher compare to other test groups. In conclusion, peptide-Ca, anchovy-Ca and MHA-Ca improved Ca bioavailability in the rats. The compounds were higher Ca digestibility compared with those of CaCO3. It is assumed that difference of digestibility for test groups may be correlated to the bioavailability of test groups in BMD, BMC, BS, BBS and bone ash respectively.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effect of Calcium Source using Tilapia Mossambica Scales on the Bone Metabolic Biomarkers and Bone Mineral Density in Rats
Gun-Ae Yoon, Kwang-Hyeon Kim
Korean J Nutr. 2010;43(4):351-356. doi: 10.4163/kjn.2010.43.4.351.
Reference
-
1. The Korean Nutrition Society. Dietary Reference Intakes for Koreans. 2005. Seoul:2. Korea Centers for Disease Control and Prevention. The third Korea National Health and Nutrition Examination Survey (KNHANES III). 2005.3. Allen LH, Wood RJ. Shils ME, Olson JA M, editors. Calcium and phosphorus. Modern Nutrition in Health and Disease. 1994. 8th Ed. 144–163.4. Allen LH. Calcium bioavailability and absorption: a review. Am J Clin Nutr. 1982. 35:783–808.
Article5. Linder MC. Linder MC, editor. Nutrition and metabolism of major minerals. Nutritional biochemistry and metabolism with clinical application. 1991. New York: Elsevier;191–214.6. Levenson DI, Bockman RS. A review of calcium preparations. Nutr Rev. 1994. 52:221–232.
Article7. Smith EL, Gilligan C, Smith PE, Sempos CT. Calcium supplementation and bone loss in middle-aged woman. Am J Clin Nutr. 1989. 50:833–842.8. Coe FL, Parks JH. Recurrent renal calcium. Cause and Prevention. Hosp Prac. 1986. 30:49–45.9. Yendt ER, Cohanim M, Jarzylo S. Reduced glomerular filtration and a renal tubular Ca leak in womae with primary osteoporosis. J Bone Min Res. 1989. 4(s):253–256.10. Sobal J, Muncie HL. Vitamin/mineral supplements use among adolescent. J Nutr Edu. 1988. 20(6):314–318.11. Korean Pharmaceutical Association. Production sheet of medicines pharmaceutics in Korea. 1995. Seoul:12. Heribert J, Watzke A. Impact of processing on bioavailability examples of minerals in foods. Trends in Food Sci & Technol. 1998. 9:320–332.
Article13. Susan J, Fairweather-Tait SJ. Forti , editor. Iron and Calcium Bioavaila-bility. Foods and Dietary Supplements. 2002. New York: International Life Sciences Institute;360–367.14. Fairweather-Tait SJ, Teucher B. Calcium bioavailability in relation to bone health. Int J Vitam Nutr Res. 2001. 72:13–18.
Article15. Gueguen L, Pointillart A. The bioavailability of dietary calcium. J Am Coll Nutr. 2000. 19:2 Suppl. 119S–136S.16. Robert PH. Factors Influencing the Measurement of Bioavailability, Taking Calcium as a Model1. 2001. Am Society for Nutritional Sciences.17. Sakhaee K, Bhuket T, Adams-Huet B, Rao DS. A meta-analysis of calcium bioavailability: a comparison of calcium citrate with calcium carbonate. Am J Ther. 1999. 6:313–321.18. Sheikh MS, Anta ACA, Nicar MJ. Gastrointestinal absorptions of calcium from milk and calcium salt. N Engl J med. 1987. 317:532–536.
Article19. Chung HK, Chang N, Lee HS, Chang YE. The effect of various types of calcium sources on calcium and bone metabolism in rats. Korean J Nutr. 1996. 29(5):480–488.20. Morohashi T, Sano T, Ohta A, Yamada S. True calcium absorption in intestine is enhanced by fructooligosaccharide feeding in rats. J Nutr. 1998. 128:1815–1818.
Article21. Harms RH, Russell GB. Adding methionine and lysine to broiler breeder diets to lower feed costs. J Appl Poult Res. 1998. 7(2):202–218.
Article22. Lewis AJ. Ammerman CB, Baker DH, Lewis AJ, editors. Bioavailability of D-amino acids and DL-Hydroxymethionine. Bioavailability of Nutrients for Animals. 1995. San Diego: Academic Press;67–81.
Article23. Turner RT. Basic Biomechanical Measurements of Bone. A Tutorial Bone. 1993. 14:595–608.24. Rath NC, Huff WE, Balog JM, Bayyari GR. Effect of gonadal steroids on bone and other physiological parameters of male broiler chickens. Poul Sci. 1996. 75:556–562.
Article25. Guéguen L, Pointillart A. The Bioavailability of Dietary Calcium. J Am Coll Nutr. 2000. 19:2 Suppl. 119S–136S.
Article26. Kwon OR, Kim MK. Effects of dietary Ca levels and kinds of lipid on the lipid metabolism in the rats. Korean J Nutr. 1988. 21(5):324–332.27. Lee JH, Moon SJ, Huh KB. Influence of phytate and low dietary calcium on calcium, phosphate and zinc metabolism by growing rats. Korean J Nutr. 1993. 26(2):145–155.28. Yacowitz H. Effects of dietary calcium upon lipid metabolism in rats fed saturated or unsaturated fat. J Nutr. 1967. 92:389–392.
Article29. Iacono JM. Effect of varying dietary level of calcium on plasma and tissue lipids of rabbits. J Nutr. 1974. 104:1165–1171.
Article30. Foley MK. Influence of dietary calcium and cholecalciferol on composition of plasma lipids in young pigs. J Nutr. 1990. 120:45–51.
Article31. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999. 14:1796–1802.
Article32. Cordey J, Schneider M, Belendez C, Ziegler WJ, Rahn BA, Perren SM. Effect of bone size, not density, on the stiffness of the proximal part of normal and osteoporotic human femora. J Bone Miner Res. 1992. 7:Suppl2. S437–S444.
Article33. Vega E, Ghiringhelli G, Mautalen C, Rey VG, Scaglia H, Zylberstein C. Bone mineral density and bone size in men with primary osteoporosis and vertebral fractures. Calcif Tissue Int. 1998. 62:465–469.
Article34. Roberfroid MB, Cumps J, Devogelaer JP. Dietary chicory inulin increases whole body bone mineral density in growing male rats. J Nutr. 2002. 132:3599–3602.
Article35. Mehta S. Bone elasticity and ultrasound velocity are affected by subtle change in organic matrix. J Bone Miner Res. 1998. 13:114–119.
Article36. Rath NC. Factors regulating bone maturity and strength in poultry. Poult Sci. 2000. 79:1024–1032.
Article37. Meulen MCH. Understanding bone strength: size isn't everything. Bone. 2001. 29:101–104.
Article38. Lee SH, Hwangbo YS, Kim JY, Lee YS. A study on the bioavailability of dietary calcium sources. Korean J Nutr. 1997. 30(5):499–505.39. Ezawa I. Studies on calcium metabolism. Jap J Home Econo. 1987. 38:695–703.40. Benson JD. Effect of previous calcium intake on adaptation to low and high calcium diets in rats. J Nutr. 1969. 97:53–60.
Article41. Sammom PE. Role of parathyroid hormone in calcium homeostasis and metabolism. Am J Physiol. 1970. 218:479–485.
Article42. Roland DA. Calcium metabolism in the laying hen: The calcium status of the hen at night. Poult Sci. 1973. 52:351–354.43. Matkovic V. Factors that influence peak bone mass formation: A study of calcium balance and the inheritance of bone mass in adolescent females. Am J Clin Nutr. 1990. 52:878–888.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Calcium Bioavailability of Eggshell Powder in the Growing Rats
- Evaluation of fat sources (lecithin, mono-glyceride and mono-diglyceride) in weaned pigs: Apparent total tract and ileal nutrient digestibilities
- The Effect of Excess Calcium on the Iron Bioavailability and Bone Growth of Marginally Iron Deficient Rats
- Bone Health and Calcium, Vitamin D, Potassium: Shortfall Nutrients in Korean
- The stable isotope method for determining absolute bioavailability