Ann Rehabil Med.
2012 Feb;36(1):159-162. 10.5535/arm.2012.36.1.159.
Cefepime Neurotoxicity in Patients with Renal Insufficiency
- Affiliations
-
- 1Department of Rehabilitation Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul 143-729, Korea. mdlis@nate.com
- KMID: 2266796
- DOI: http://doi.org/10.5535/arm.2012.36.1.159
Abstract
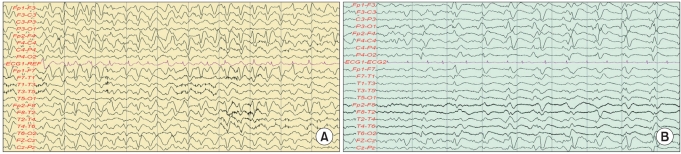
- Cefepime is a fourth-generation cephalosporin that is active against both gram-positive and gram-negative organisms. It is administered parenterally for the treatment of severe infections. Approximately 85% of the drug is excreted unchanged by the kidneys. Neurotoxicity in patients with renal failure who are treated with cefepime has been reported sporadically. We report on two senile patients with renal impairment who developed neurotoxicity including lethal outcome after treatment with cefepime.
Keyword
Figure
Reference
-
1. Rybak M. The pharmacokinetic profile of a new generation of parenteral cephalosporin. Am J Med. 1996; 100:39S–44S. PMID: 8678096.
Article2. Sonck J, Laureys G, Verbeelen D. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol Dial Transplant. 2008; 23:966–970. PMID: 18175786.
Article3. Lam S, Gomolin IH. Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review. Pharmacotherapy. 2006; 26:1169–1174. PMID: 16863493.
Article4. Fernandez-Torre JL. Cefepime- and cefixime-induced encephalopathy in a patient with normal renal function. Neurology. 2006; 67:367. PMID: 16864850.
Article5. Chow KM, Hui AC, Szeto CC. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis. 2005; 24:649–653. PMID: 16261307.
Article6. Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis. 2007; 7:338–348. PMID: 17448937.
Article7. Neu HC. Safety of cefepime: a new extended-spectrum parenteral cephalosporin. Am J Med. 1996; 100:68S–75S. PMID: 8678100.
Article8. Chatellier D, Jourdain M, Mangalaboyi J, Ader F, Chopin C, Derambure P, Fourrier F. Cefepime-induced neurotoxicity: an underestimated complication of antibiotherapy in patients with acute renal failure. Intensive Care Med. 2002; 28:214–217. PMID: 11907668.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cefepime-induced neurotoxicity
- Cefepime-induced neurotoxicity after flap surgery: a rare case report
- Neurotoxicity Induced by Cefepime in a Patient with Minimal Change Disease
- Cefepime-induced nonconvulsive status epilepticus in a hemodialysis patient
- Cefepime-Induced Reversible Encephalopathy with Triphasic Waves in Patients with Impaired Renal Function