Ann Dermatol.
2013 Aug;25(3):310-314. 10.5021/ad.2013.25.3.310.
Evaluation of Expression of Lipases and Phospholipases of Malassezia restricta in Patients with Seborrheic Dermatitis
- Affiliations
-
- 1Department of Dermatology, School of Medicine, Konkuk University, Seoul, Korea.
- 2Department of Systems Biotechnology, Chung-Ang University, Anseong, Korea. whjung@cau.ac.kr
- 3Department of Chemistry, KAIST, Daejeon, Korea.
- KMID: 2265870
- DOI: http://doi.org/10.5021/ad.2013.25.3.310
Abstract
- BACKGROUND
Malassezia species (spp.) are cutaneous opportunistic pathogens and associated with various dermatological diseases including seborrheic dermatitis, dandruff and atopic dermatitis. Almost all Malassezia spp. are obligatorily lipid-dependent, which might be caused by lack of the myristic acid synthesis. Recent genome analysis of M. restricta and M. globosa suggested that the absence of a gene encoding fatty acid synthesis might be compensated by abundant genes encoding hydrolases, which produce fatty acids, and that lipases and phospholipases may play a role in virulence of the fungus.
OBJECTIVE
The current study aimed to investigate the contribution of lipases and phospholipases in virulence of the M. restricta as being the most frequently isolated Malassezia spp. from the human skin.
METHODS
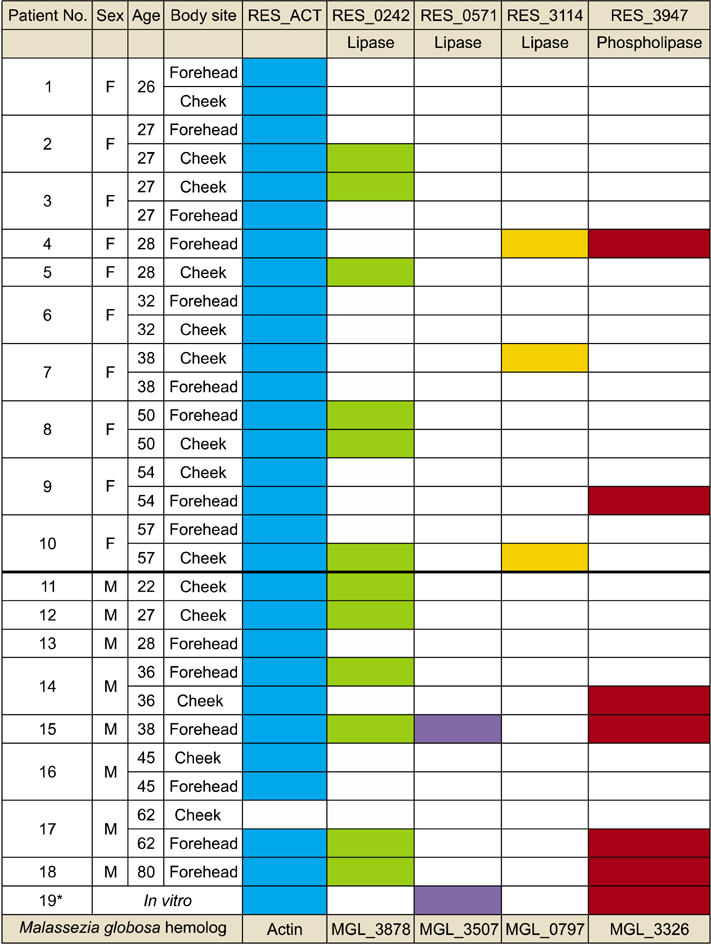
Swap samples of two different body sites of at least 18 patients with seborrheic dermatitis were obtained and in vivo expression of lipases and phospholipases of M. restricta was analyzed by the gene specific two-step nested RT-PCR.
RESULTS
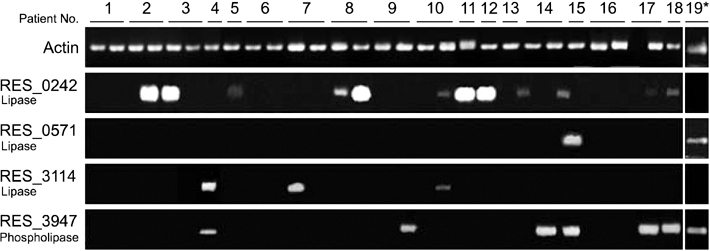
The results of the current study suggest that majority of the patients display expression of lipase RES_0242.
CONCLUSION
These data imply a possible role of lipase in the host environment to produce free fatty acids for the fungus.
MeSH Terms
Figure
Cited by 1 articles
-
Progress in Malassezia Research in Korea
Soo Young Kim, Yang Won Lee, Yong Beom Choe, Kyu Joong Ahn
Ann Dermatol. 2015;27(6):647-657. doi: 10.5021/ad.2015.27.6.647.
Reference
-
1. Ashbee HR. Recent developments in the immunology and biology of Malassezia species. FEMS Immunol Med Microbiol. 2006; 47:14–23.2. Hort W, Mayser P. Malassezia virulence determinants. Curr Opin Infect Dis. 2011; 24:100–105.
Article3. Lee YW, Yim SM, Lim SH, Choe YB, Ahn KJ. Quantitative investigation on the distribution of Malassezia species on healthy human skin in Korea. Mycoses. 2006; 49:405–410.
Article4. Thompson E, Colvin JR. Composition of the cell wall of Pityrosporum ovale (Bizzozero) Castellani and Chalmers. Can J Microbiol. 1970; 16:263–265.
Article5. DeAngelis YM, Saunders CW, Johnstone KR, Reeder NL, Coleman CG, Kaczvinsky JR Jr, et al. Isolation and expression of a Malassezia globosa lipase gene, LIP1. J Invest Dermatol. 2007; 127:2138–2146.
Article6. Xu J, Saunders CW, Hu P, Grant RA, Boekhout T, Kuramae EE, et al. Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc Natl Acad Sci U S A. 2007; 104:18730–18735.
Article7. Brunke S, Hube B. MfLIP1, a gene encoding an extracellular lipase of the lipid-dependent fungus Malassezia furfur. Microbiology. 2006; 152:547–554.
Article8. Juntachai W, Oura T, Murayama SY, Kajiwara S. The lipolytic enzymes activities of Malassezia species. Med Mycol. 2009; 47:477–484.
Article9. Plotkin LI, Squiquera L, Mathov I, Galimberti R, Leoni J. Characterization of the lipase activity of Malassezia furfur. J Med Vet Mycol. 1996; 34:43–48.10. Riciputo RM, Oliveri S, Micali G, Sapuppo A. Phospholipase activity in Malassezia furfur pathogenic strains. Mycoses. 1996; 39:233–235.11. Lee YW, Byun HJ, Kim BJ, Kim DH, Lim YY, Lee JW, et al. Distribution of malassezia species on the scalp in korean seborrheic dermatitis patients. Ann Dermatol. 2011; 23:156–161.
Article12. Ibrahim AS, Mirbod F, Filler SG, Banno Y, Cole GT, Kitajima Y, et al. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect Immun. 1995; 63:1993–1998.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Malassezia Species Cultured From the Lesions of Seborrheic Dermatitis
- Malassezia Species Cultured from the Lesions of Malassezia Folliculitis
- Distribution of Malassezia Species on the Scalp in Korean Seborrheic Dermatitis Patients
- Epidemiologic Study of Malassezia Yeasts in Seborrheic Dermatitis Patients by the Analysis of 26S rDNA PCR-RFLP
- A Case of Seborrheic Dermatitis Caused by Overgrowth of Malassezia globosa