J Korean Ophthalmol Soc.
2014 Oct;55(10):1520-1524. 10.3341/jkos.2014.55.10.1520.
Side Effects after the Use of Cyclopentolate for Cycloplegic Refraction
- Affiliations
-
- 1Department of Ophthalmology, Hallym University College of Medicine, Anyang, Korea. Ljy690725@hanmail.net
- KMID: 2216872
- DOI: http://doi.org/10.3341/jkos.2014.55.10.1520
Abstract
- PURPOSE
To investigate the frequency of side effects due to the use of cyclopentolate for cycloplegic refraction.
METHODS
For 4 months, temperature change and adverse effects in 157 children who visited the pediatric ophthalmology clinic of a university hospital for cycloplegic refraction using cyclopentolate were observed. Topical 1% cyclopentolate was instilled 5 times at 5 minute intervals and temperature measured before and after administration using a tympanic thermometer. Side effects such as facial flushing, skin rash, and central nervous system disorders were recorded while cycloplegic refraction was performed.
RESULTS
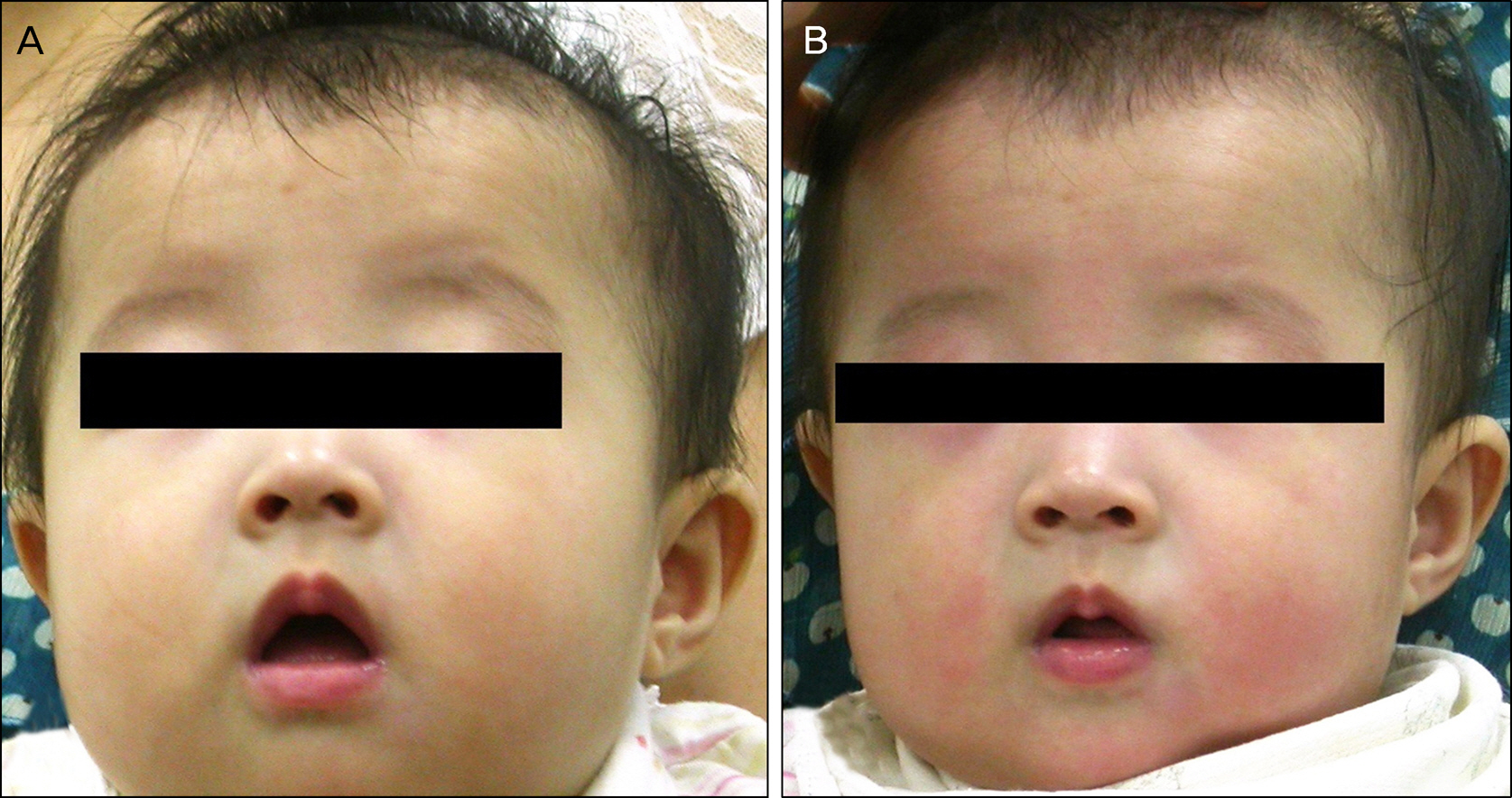
The mean temperature was increased 36.67 +/- 0.10degrees C to 36.90 +/- 0.09degrees C, but no fever exceeded 38degrees C. Seventeen (10.83%) patients experienced side effects including facial flushes (n = 6), temperature change (n = 5), abnormal central nervous system symptoms (n = 4), and a visible skin rash (n = 2). No patient experienced more than 1 side effect and long term adverse effects were not observed.
CONCLUSIONS
The incidence of side effects due to cyclopentolate instillation for cycloplegic refraction was 10.83% in children. Although side effects due to cyclopentolate disappeared without any treatment, cafeful monitoring for their occurrence is necessary.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Moon NJ, Kim JC, Koo BS. The study on the necessity of cycloplegic refraction in school children. J Korean Ophthalmol Soc. 1988; 29:377–85.2. Benjamin M, Melvin LR. The fine art of prescribing glasses without making a spectacle of yourself. 2nd ed.Triad Publishing Company;1991. p. 56.3. Cho BC, Kim YH, Choi KR. The effects of cycloplegic (cyclogyl) on refractive states. J Korean Ophthalmol Soc. 1985; 26:293–301.4. Shin KM, Chung SA, Lee JB. Comparative study on the efficacy of different cycloplegic agents in myopic adults. J Korean Ophthalmol Soc. 2011; 52:141–6.
Article5. Arthur GB, Ronald BR. Clinical visual optics. 2nd ed.Butterworths;1989. p. 133.6. Jimmy DB, Siret DJ. Clinical ocular pharmacology. 2nd ed.Butterworth;1989. p. 132–4.7. Mirshahi A, Kohnen T. Acute psychotic reaction caused by topical cyclopentolate use for cycloplegic refraction before refractive surgery: case report and review of the literature. J Cataract Refract Surg. 2003; 29:1026–30.8. Bhatia SS, Vidyashankar C, Sharma RK, Dubey AK. Systemic toxicity with cyclopentolate eye drops. Indian Pediatr. 2000; 37:329–31.9. Elibol O, Alçelik T, Yüksel N, Caglar Y. The influence of drop size of cyclopentolate, phenylephrine and tropicamide on pupil dilatation and systemic side effects in infants. Acta Ophthalmol Scand. 1997; 75:178–80.
Article10. Kliegman RM, Stanton BF, St. Geme JW III, et al. Nelson textbook of pediatrics. 19th ed.Elsevier;2011. chap. 169.11. Laszlo G. Pharmacology of antimuscarinic agents. 47:CRC Press;1997. p. 136–7.12. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007; 9:191–6.13. Gray LG. Avoiding adverse effects of cycloplegics in infants and children. J Am Optom Assoc. 1979; 50:465–70.14. Vale J, Cox B. Drugs and the Eye. Butterworths. 1978; 21.15. Palmer EA. How safe are ocular drugs in pediatrics? Ophthalmology. 1986; 93:1038–40.
Article16. Rozette NA, Matragoon S, Sethi S, et al. Systemic effects of ophthalmic cyclopentolate on body weight in neonatal mice. Neonatology. 2014; 106:37–41.
Article17. Lahdes K, Huupponen R, Kaila T, et al. Systemic absorption of ocular cyclopentolate in children. Ger J Ophthalmol. 1992; 1:16–8.18. Derinoz O, Emeksiz HC. Use of physostigmine for cyclopentolate overdose in an infant. Pediatrics. 2012; 130:e703–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Statistic Observation About Effect of Cycloplegic Refraction in Primary School Children
- Cycloplegic Refraction in Esotropic Children: Cydopentolate versus Atropine
- Comparative Study on the Efficacy of Different Cycloplegic Agents in Myopic Adults
- Cycloplegic Refraction in Hyperopic Children: Effectiveness of a 0.5% Tropicamide and 0.5% Phenylephrine Addition to 1% Cyclopentolate Regimen
- Usefulness of Cycloplegic Refraction with Atropine in Patients with Partially Accommodative Esotropia