J Korean Ophthalmol Soc.
2011 Dec;52(12):1507-1513. 10.3341/jkos.2011.52.12.1507.
Mechanisms Underlying Trabecular Meshwork Cell Death Caused by Mutant Myocilin Expression
- Affiliations
-
- 1Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. cw.kee@samsung.com
- 2Center for Clinical Research, Samsung Biomedical Research Institute, Seoul, Korea.
- KMID: 2215157
- DOI: http://doi.org/10.3341/jkos.2011.52.12.1507
Abstract
- PURPOSE
To determine whether the expression of mutant myocilin can lead to death of human trabecular meshwork (HTM) cells and to determine whether the mechanism by which this occurs is apoptosis.
METHODS
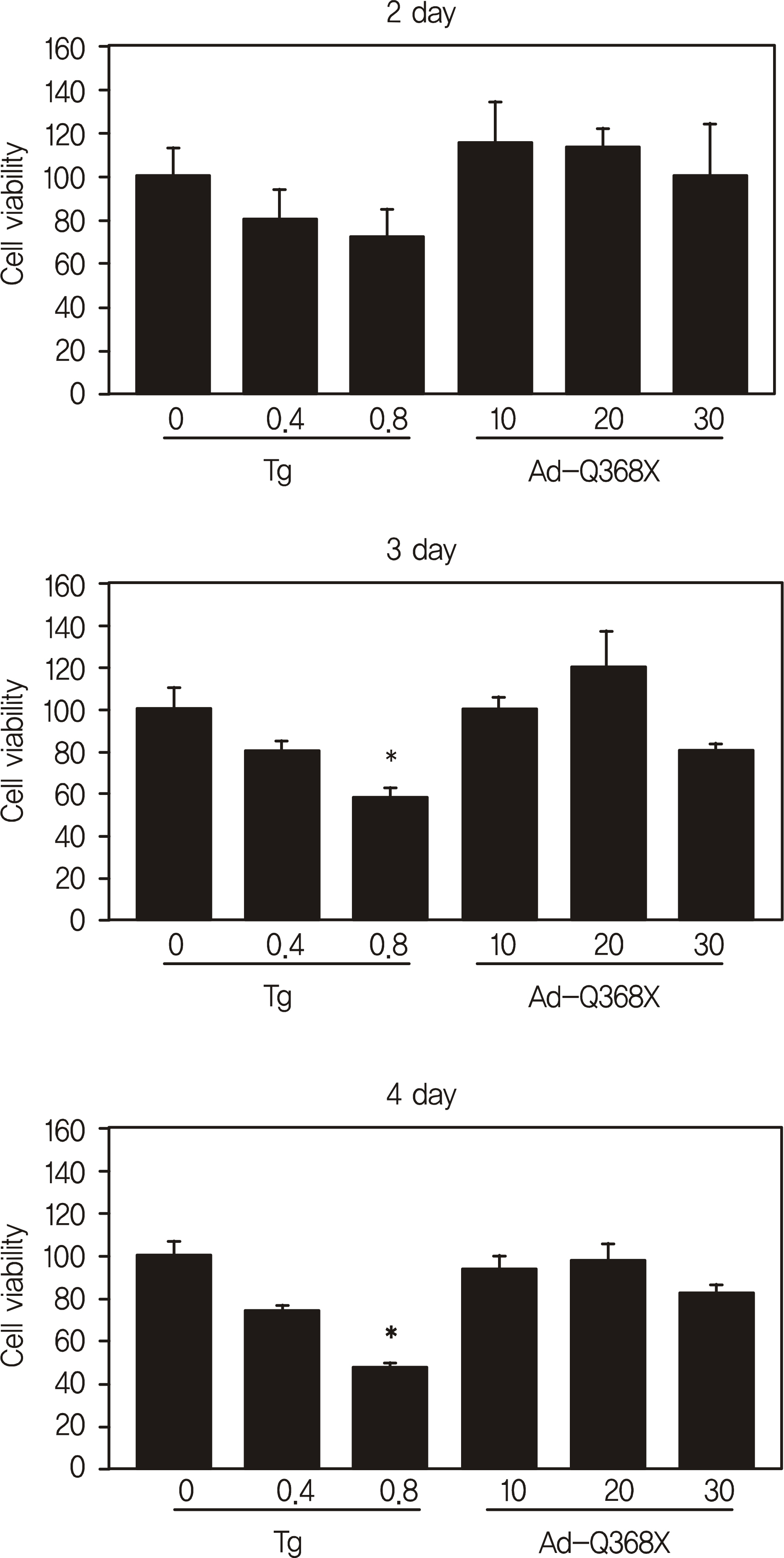
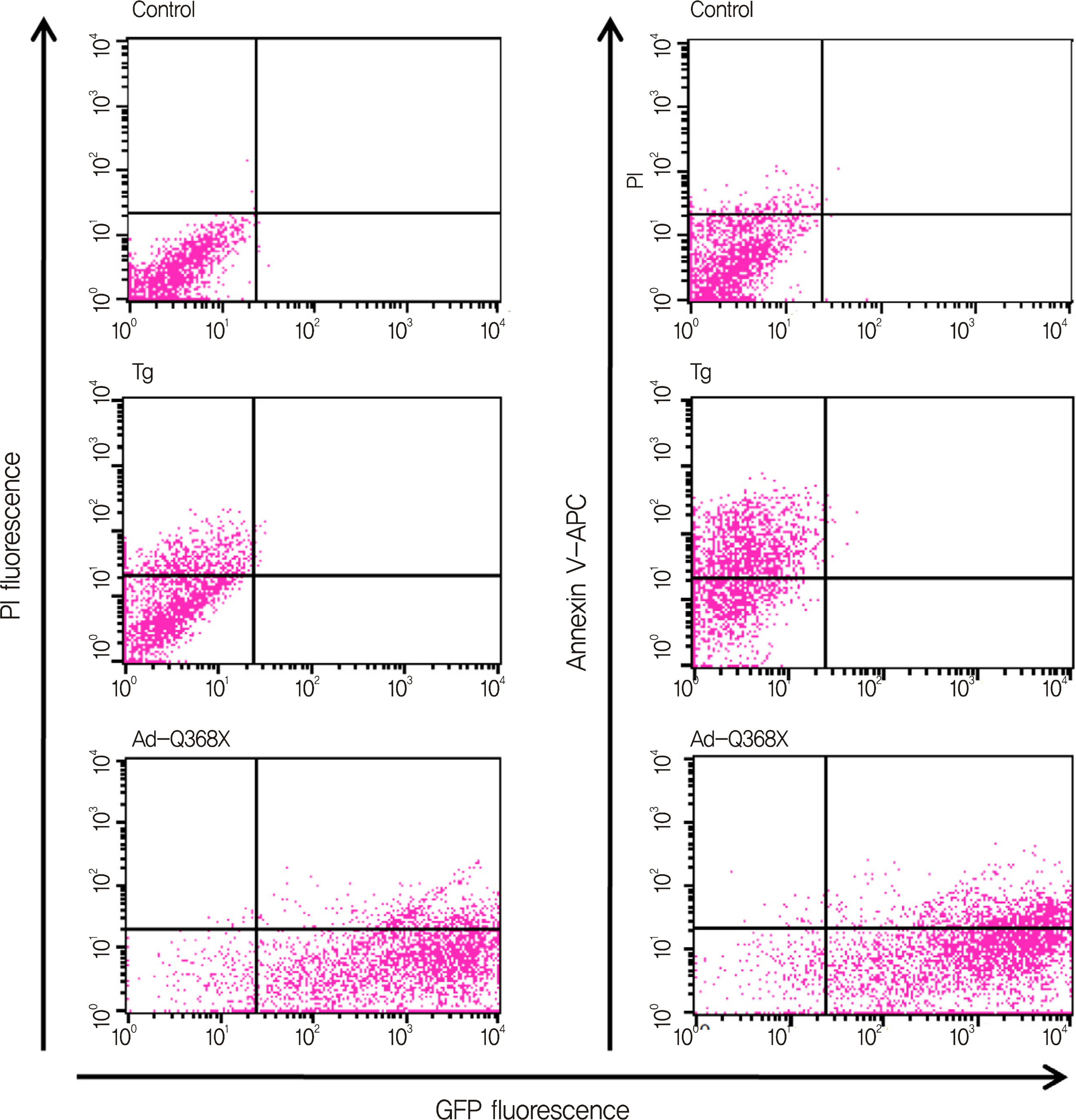
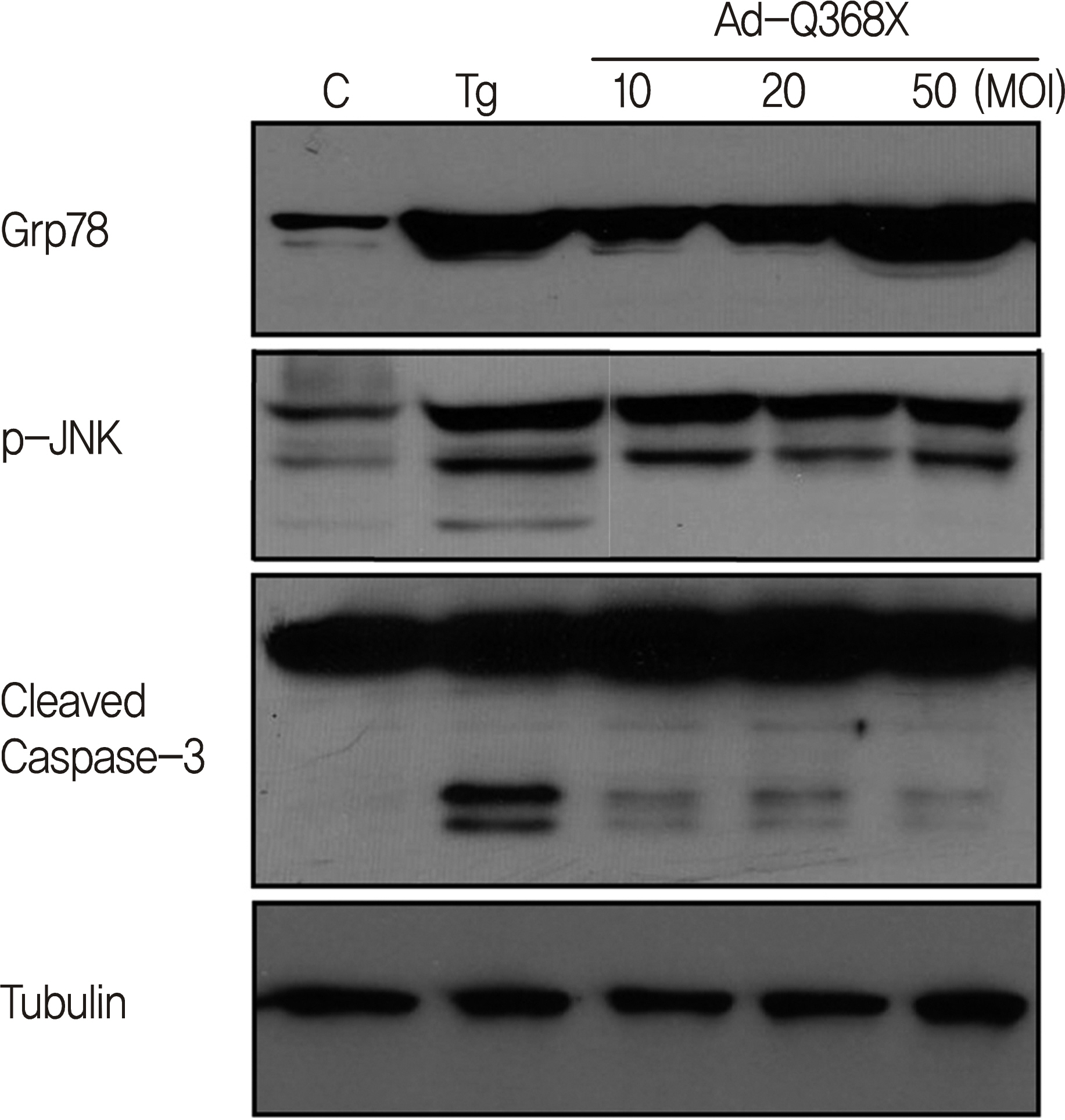
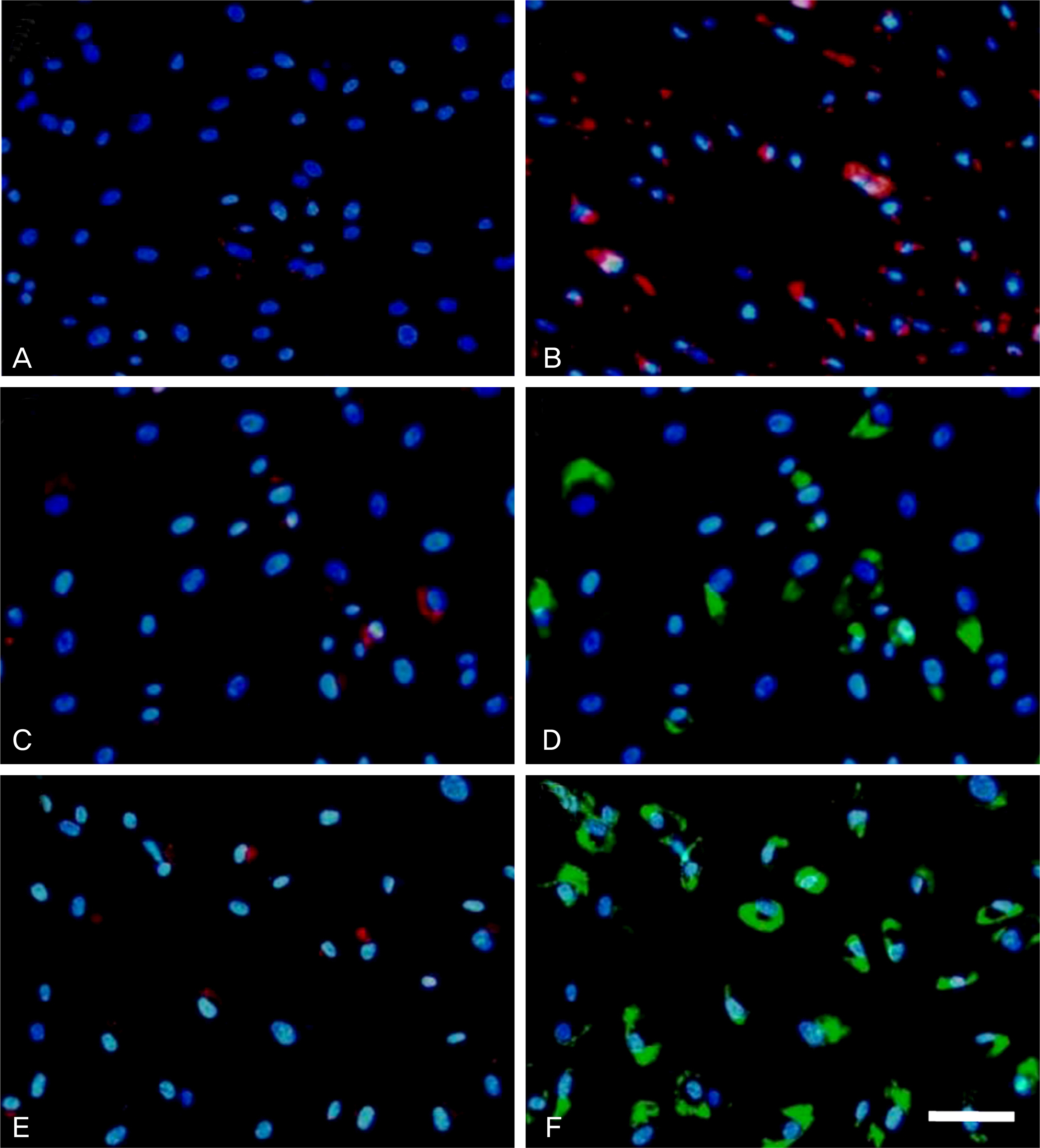
HTM cells were transduced with a recombinant adenovirus expressing human mutant (Q368X) myocilin. The apoptotic death of HTM cells caused by expression of mutant myocilin was examined using a cell proliferation assay, flow cytometry, Western blot analysis, and immunocytochemistry.
RESULTS
It appeared that the expression of mutant myocilin itself was not sufficient to cause HTM cell death. Furthermore, the expression of mutant myocilin did not lead to apoptosis of HTM cells although it did elicit a protein unfolding response.
CONCLUSIONS
Our data suggest that the mechanism of myocilin glaucoma is not apoptotic death of HTM cells caused by mutant myocilin expression, and that the actual mechanism remains unknown.
MeSH Terms
Figure
Reference
-
References
1. Stone EM, Fingert JH, Alward WLM, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997; 275:668–70.
Article2. Polansky JR, Kurtz RM, Alvarado JA, et al. Eicosanoid production and glucocorticoid regulatory mechanisms in cultured human trabecular meshwork cells. Prog Clin Biol Res. 1989; 312:113–38.3. Nguyen TD, Chen P, Huang WD, et al. Gene structure and proper-ties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998; 273:6341–50.
Article4. Sohn S, Hur W, Joe MK, et al. Expression of wild-type and trun-cated myocilins in trabecular meshwork cells: their subcellular lo-calizations and cytotoxicities. Invest Ophthalmol Vis Sci. 2002; 43:3680–5.5. Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002; 47:547–61.
Article6. Alward WL, Fingert JH, Coote MA, et al. Clinical features associated with mutations in the chromosome 1 openangle glaucoma gene (GLC1A). N Engl J Med. 1998; 338:1022–7.
Article7. Fingert JH, Heon E, Liebmann JM, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999; 8:899–905.
Article8. Yoon SJ, Kim HS, Moon JI, et al. Mutations of the TIGR/MYOC gene in primary openangle glaucoma in Korea. Am J Hum Genet. 1999; 64:1775–8.
Article9. Wiggs JL, Vollrath D. Molecular and clinical evaluation of a patient hemizygous for TIGR/MYOC. Arch Ophthalmol. 2001; 119:1674–8.
Article10. Lam DS, Leung YF, Chua JK, et al. Truncations in the TIGR gene in individuals with and without primary openangle glaucoma. Invest Ophthalmol Vis Sci. 2000; 41:1386–91.11. Kim BS, Savinova OV, Reedy MV, et al. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol Cel Biol. 2001; 21:7707–13.12. Zhou ZH, Vollrath D. A cellular assay distinguishes normal and mutant TIGR/myocilin protein. Hum Mol Genet. 1999; 8:2221–8.
Article14. Jacobson N, Andrews M, Shepard AR, et al. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001; 10:117–25.
Article15. Joe MK, Sohn S, Hur W, et al. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003; 312:592–600.
Article16. Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004; 13:1193–204.
Article17. Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Aggregated myocilin induces Russell bodies and causes apoptosis - Implications for the pathogenesis of myocilin-caused primary openangle glaucoma. Am J Pathol. 2007; 170:100–9.18. Karali A, Russell P, Stefani FH, Tamm ER. Localization of myocilin/trabecular meshwork–inducible glucocorticoid response protein in the human eye. Invest Ophthalmol Vis Sci. 2000; 41:729–40.19. Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol. 2010; 189:783–94.
Article20. Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006; 11:5–13.
Article21. Moretti L, Cha YI, Niermann KJ, Lu B. Switch between apoptosis and autophagy: radiation-induced endoplasmic reticulum stress? Cell Cycle. 2007; 6:793–8.
Article22. Joe MK, Tomarev SI. Expression of myocilin mutants sensitizes cells to oxidative stress-induced apoptosis: implication for glaucoma pathogenesis. Am J Pathol. 2010; 176:2880–90.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Cytotoxicity of Truncated (Q368X) Myocilin in Trabecular Meshwork (TM) Cells
- The Effect of Accumulation of Mutant Myocilins in Human Trabecular Meshwork Cells
- Expression of Wild Type and Truncated Myocilins in Trabecular Meshwork Cells: their Subcellular Localization
- Effects of Vascular Endothelial Growth Factor on YAP/TAZ Signaling in Trabecular Meshwork Cells
- Screening and Selection of Proteins Interacting with Myocilin