J Korean Surg Soc.
2013 Apr;84(4):202-208. 10.4174/jkss.2013.84.4.202.
Successful mouse hepatocyte culture with sandwich collagen gel formation
- Affiliations
-
- 1Department of Surgery, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea. dhchoi@schmc.ac.kr
- KMID: 2212464
- DOI: http://doi.org/10.4174/jkss.2013.84.4.202
Abstract
- PURPOSE
Primary mammalian hepatocytes largely retain their liver-specific functions when they are freshly derived from donors. However, long-term cultures of functional hepatocytes are difficult to establish. To increase the longevity and maintain the differentiated functions of hepatocytes in primary culture, cells can be cultured in a sandwich configuration of collagen. In such a configuration, hepatocytes can be cultured for longer periods compared with cultures on single layers of collagen. However, research regarding mouse hepatocytes in sandwich culture is lacking.
METHODS
Primary mouse hepatocytes were sandwiched between two layers of collagen to maintain the stability of their liver-specific functions. After gelation, 2 mL of hepatocyte culture medium was applied.
RESULTS
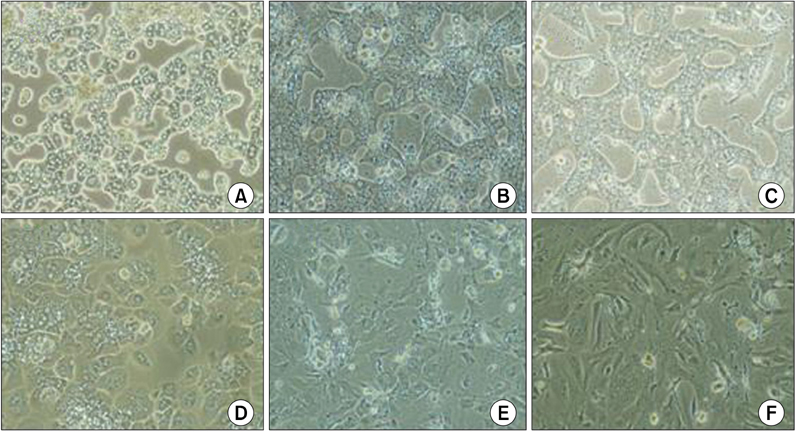
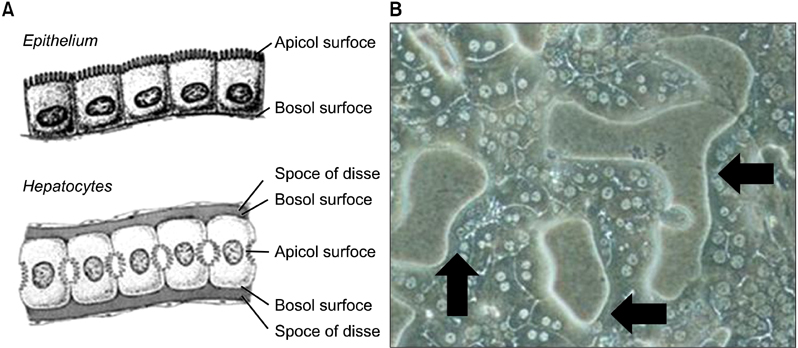
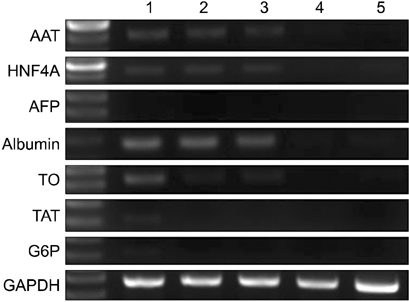
After 24 hours, 5, 10 days of culture, the collagen gel sandwich maintained the cellular border and numbers of bile canaliculi more efficiently than a single collagen coating in both high and low density culture dishes. Reverse transcription-polymerase chain reaction analysis of alpha-1-antitrypsin (AAT), hepatocyte nuclear factor 4 alpha (HNF4A), alphafetoprotein, albumin, tryptophan oxygenase (TO), the tyrosine aminotransferase gene, glucose-6-phosphatase, glyceraldehyde-3-phosphate dehydrogenase for mouse primary hepatocytes cultured on collagen coated dishes and collagen gels showed superior hepatocyte-related gene expression in cells grown using the collagen gel sandwich culture system. AAT, HNF4A, albumin, TO were found to be expressed in mouse hepatocytes cultured on collagen gels for 5 and 10 days. In contrast, mouse hepatocytes grown on collagen-coated dishes did not express these genes after 5 and 10 days of culture.
CONCLUSION
The collagen gel sandwich method is suitable for primary culture system of adult mouse hepatocytes.
Keyword
MeSH Terms
-
Adult
Animals
Bile Canaliculi
Collagen
Gels
Gene Expression
Glucose-6-Phosphatase
Hepatocyte Nuclear Factor 4
Hepatocytes
Humans
Longevity
Mice
Oxidoreductases
Tissue Donors
Tryptophan Oxygenase
Tyrosine Transaminase
Collagen
Gels
Glucose-6-Phosphatase
Hepatocyte Nuclear Factor 4
Oxidoreductases
Tryptophan Oxygenase
Tyrosine Transaminase
Figure
Cited by 1 articles
-
Cell Sources, Liver Support Systems and Liver Tissue Engineering: Alternatives to Liver Transplantation
Soo Young Lee, Han Joon Kim, Dongho Choi
Int J Stem Cells. 2015;8(1):36-47. doi: 10.15283/ijsc.2015.8.1.36.
Reference
-
1. Wang YJ, Liu HL, Guo HT, Wen HW, Liu J. Primary hepatocyte culture in collagen gel mixture and collagen sandwich. World J Gastroenterol. 2004. 10:699–702.2. Swift B, Brouwer KL. Influence of seeding density and extracellular matrix on bile Acid transport and mrp4 expression in sandwich-cultured mouse hepatocytes. Mol Pharm. 2010. 7:491–500.3. Mathijs K, Kienhuis AS, Brauers KJ, Jennen DG, Lahoz A, Kleinjans JC, et al. Assessing the metabolic competence of sandwich-cultured mouse primary hepatocytes. Drug Metab Dispos. 2009. 37:1305–1311.4. Martinez SM, Bradford BU, Soldatow VY, Kosyk O, Sandot A, Witek R, et al. Evaluation of an in vitro toxicogenetic mouse model for hepatotoxicity. Toxicol Appl Pharmacol. 2010. 249:208–216.5. Berthiaume F, Tompkins RG, Yarmush ML. Isolation and long-term maintenance of adult rat hepatocytes in culture. Methods Mol Med. 1999. 18:447–456.6. Sattler CA, Michalopoulos G, Sattler GL, Pitot HC. Ultrastructure of adult rat hepatocytes cultured on floating collagen membranes. Cancer Res. 1978. 38:1539–1549.7. Lang R, Stern MM, Smith L, Liu Y, Bharadwaj S, Liu G, et al. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials. 2011. 32:7042–7052.8. Lee CH, Edwards AM. Stimulation of DNA synthesis by tumor promoters in primary rat hepatocytes is not mediated by arachidonic acid metabolites. J Cell Physiol. 2001. 187:336–344.9. Kost DP, Michalopoulos GK. Effect of 2% dimethyl sulfoxide on the mitogenic properties of epidermal growth factor and hepatocyte growth factor in primary hepatocyte culture. J Cell Physiol. 1991. 147:274–280.10. Diao L, Li N, Brayman TG, Hotz KJ, Lai Y. Regulation of MRP2/ABCC2 and BSEP/ABCB11 expression in sandwich cultured human and rat hepatocytes exposed to inflammatory cytokines TNF-{alpha}, IL-6, and IL-1{beta}. J Biol Chem. 2010. 285:31185–31192.11. Yin J, Meng Q. Use of primary rat hepatocytes in the gel entrapment culture to predict in vivo biliary excretion. Xenobiotica. 2012. 42:417–428.12. Liu X, Chism JP, LeCluyse EL, Brouwer KR, Brouwer KL. Correlation of biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats. Drug Metab Dispos. 1999. 27:637–644.13. Marion TL, Perry CH, St Claire RL 3rd, Brouwer KL. Endogenous bile acid disposition in rat and human sandwich-cultured hepatocytes. Toxicol Appl Pharmacol. 2012. 261:1–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Collagen Gel Contraction by Cultured Fibroblasts Derived from Normal Skin, Oral Mucosa, and Hypertrophic Scar
- Growth and Differentiation of Preadipocytes in Alginate and Collagen Gels
- Hepatocyte Isolation, Culture, and Its Clinical Applications
- Change of the Effect of TGF-beta1 on Physeal Chondrocytes According to Culture Methods in Vitro
- Transplantation of Culture-Expanded Bone Marrow Mesenchymal Cells and Type I Collagen Gel-Xenograft Complex