J Korean Soc Magn Reson Med.
2010 Dec;14(2):115-120. 10.13104/jksmrm.2010.14.2.115.
Low Frequency Fluctuation Component Analysis in Active Stimulation fMRI Paradigm
- Affiliations
-
- 1Department of Medical and Biological Engineering, Kyungpook National University, Korea.
- 2Jeju National University College of Veterinary Medicine, Korea.
- 3Department of Radiology, Kyungpook National University Hospital, Korea.
- KMID: 2206908
- DOI: http://doi.org/10.13104/jksmrm.2010.14.2.115
Abstract
- PURPOSE
To separate and evaluate the low frequency spontaneous fluctuation BOLD signals from the functional magnetic resonance imaging data using sensorimotor active task.
MATERIALS AND METHODS
Twenty female archery players and twenty three control subjects were included in this study. Finger-tapping task consisted of three cycles of right finger tapping, with a subsequent 30 second rest. Blood oxygenation level-dependent (BOLD) data were collected using T2*-weighted echo planar imaging at a 3.0 T scanner. A 3-D FSPGR T1-weighted images were used for structural reference. Image processing and statistical analyses were performed using SPM5 for active finger-tapping task and GIFT program was used for statistical analyses of low frequency spontaneous fluctuation BOLD signal.
RESULTS
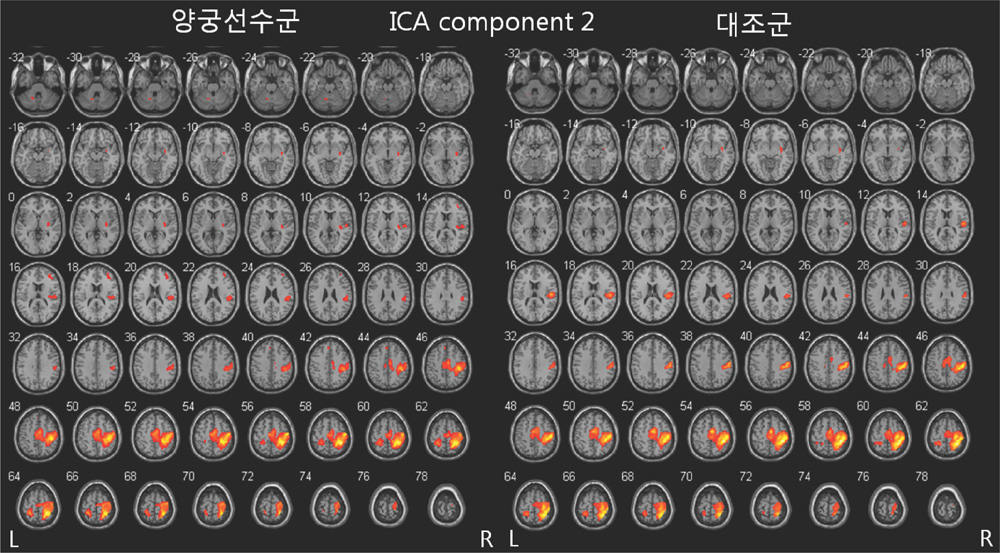
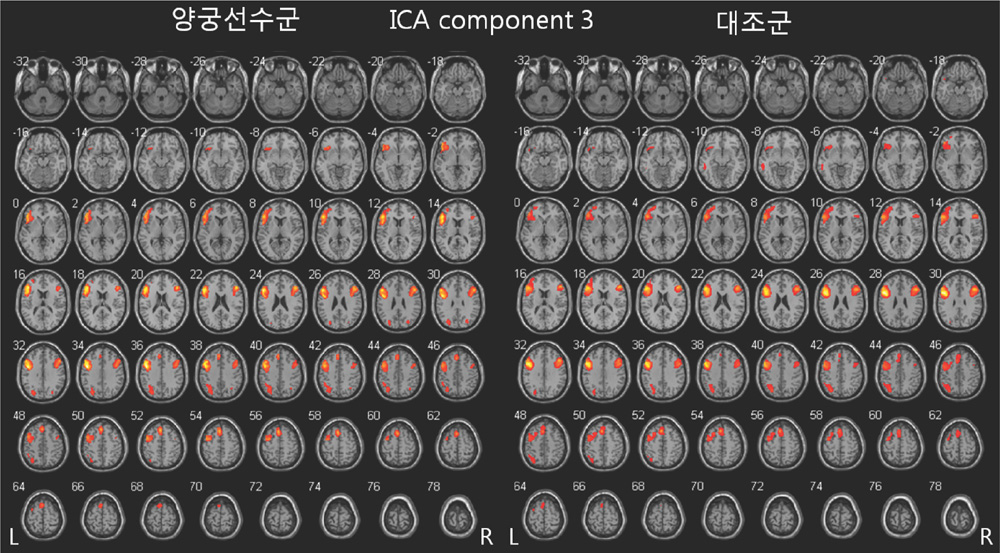
Both groups showed the activation in the left primary motor cortex and supplemental motor area and in the right cerebellum for right finger-tapping task. ICA analysis using GIFT revealed independent components corresponding to contralateral and ipsilateral sensorimotor network and cognitive-related neural network.
CONCLUSION
The current study demonstrated that the low frequency spontaneous fluctuation BOLD signals can be separated from the fMRI data using finger tapping paradigm. Also, it was found that these independent components correspond to spontaneous and coherent neural activity in the primary sensorimotor network and in the motor-cognitive network.
Keyword
MeSH Terms
Figure
Reference
-
1. Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain-function during task activation. Magn Reson Med. 1992. 25:390–397.2. Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation-functional brain mapping with magnetic-resonance-imaging. Proc Natl Acad Sci USA. 1992. 89:5951–5955.3. Attwell D, Laughlin SB. An energy budget for signalling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001. 21:1133–1145.4. Ames AI. CNS energy metabolism as related to function. Brain Res Rev. 2000. 34:42–68.5. Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006. 29:449–476.6. Indovina I, Sanes JN. Combined visual attention and finger movement effects on human brain representations. Exp Brain Res. 2001. 140:265–279.7. Sadato N, Campbell G, Ibáñez V, Deiber M, Hallett M. Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci. 1996. 16:2691–2700.8. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain. Magn Reson Med. 1995. 34:537–541.9. Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a metaanalysis. Neuroimage. 2006. 31:1453–1474.10. Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991. 66:705–718.11. Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002. 15:373–385.12. Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol. 1999. 81:3065–3077.13. Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000. 123:1216–1228.14. Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007. 45(13):2883–2901.15. Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006. 15:1536–1548.16. Shmueli K, van Gelderen P, de Zwart JA, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007. 38:306–320.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of fMRI Signal Using Independent Component Analysis
- Applications of Functional Magnetic Resonance Imaging(fMRI) to the Research of Psychiatric Disorders
- Severity of Obstructive Sleep Apnea and Heart Rate Variability : Detrended Fluctuation Analysis
- Alterations in Spontaneous Brain Activity in Drug-Naïve First-Episode Schizophrenia: An Anatomical/Activation Likelihood Estimation Meta-Analysis
- A Preliminary Results of Acoustic Noise Effect due to Gradient Pulsing in Functional MRI