Restor Dent Endod.
2015 Aug;40(3):195-201. 10.5395/rde.2015.40.3.195.
Chelating and antibacterial properties of chitosan nanoparticles on dentin
- Affiliations
-
- 1Department of Dentistry, Endodontics and Dental Materials, Bauru Dental School, University of Sao Paulo, Bauru-Sao Paulo, Brazil. aldodelcp@usp.br
- 2Department of Physics and Chemistry, FEIS, Sao Paulo State University, Ilha Solteira-Sao Paulo, Brazil.
- 3National Nanotechnology Laboratory for Agriculture, Embrapa, Sao Carlos-Sao Paulo, Brazil.
- 4Discipline of Endodontics, Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada.
- KMID: 2396462
- DOI: http://doi.org/10.5395/rde.2015.40.3.195
Abstract
OBJECTIVES
The use of chitosan nanoparticles (CNPs) in endodontics is of interest due to their antibiofilm properties. This study was to investigate the ability of bioactive CNPs to remove the smear layer and inhibit bacterial recolonization on dentin.
MATERIALS AND METHODS
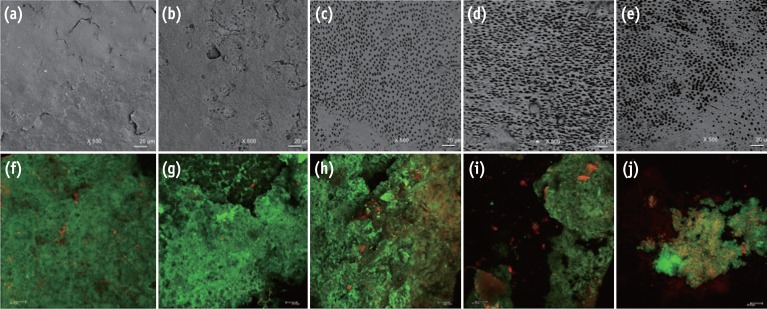
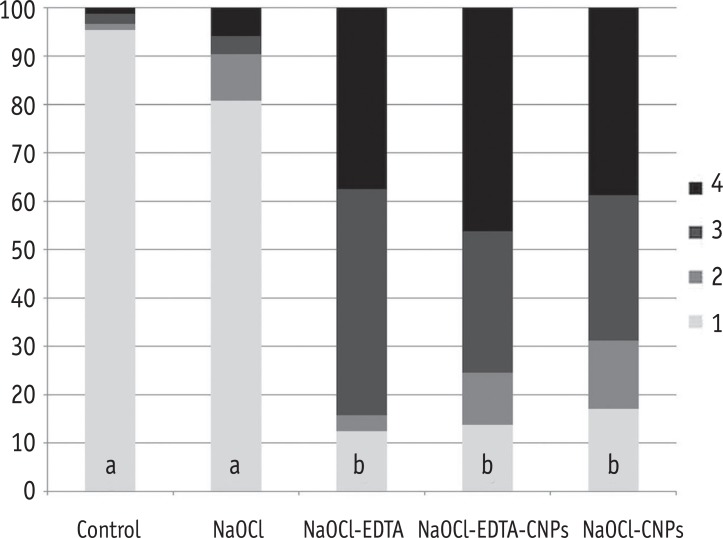
One hundred bovine dentin sections were divided into five groups (n = 20 per group) according to the treatment. The irrigating solutions used were 2.5% sodium hypochlorite (NaOCl) for 20 min, 17% ethylenediaminetetraacetic acid (EDTA) for 3 min and 1.29 mg/mL CNPs for 3 min. The samples were irrigated with either distilled water (control), NaOCl, NaOCl-EDTA, NaOCl-EDTA-CNPs or NaOCl-CNPs. After the treatment, half of the samples (n = 50) were used to assess the chelating effect of the solutions using portable scanning electronic microscopy, while the other half (n = 50) were infected intra-orally to examine the post-treatment bacterial biofilm forming capacity. The biovolume and cellular viability of the biofilms were analysed under confocal laser scanning microscopy. The Kappa test was performed for examiner calibration, and the non-parametric Kruskal-Wallis and Dunn tests (p < 0.05) were used for comparisons among the groups.
RESULTS
The smear layer was significantly reduced in all of the groups except the control and NaOCl groups (p < 0.05). The CNPs-treated samples were able to resist biofilm formation significantly better than other treatment groups (p < 0.05).
CONCLUSIONS
CNPs could be used as a final irrigant during root canal treatment with the dual benefit of removing the smear layer and inhibiting bacterial recolonization on root dentin.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Analysis of the shelf life of chitosan stored in different types of packaging, using colorimetry and dentin microhardness
Antonio Miranda da Cruz-Filho, Angelo Rafael de Vito Bordin, Luis Eduardo Souza-Flamini, Débora Fernandes da Costa Guedes, Paulo César Saquy, Ricardo Gariba Silva, Jesus Djalma Pécora
Restor Dent Endod. 2017;42(2):87-94. doi: 10.5395/rde.2017.42.2.87.Enhanced visualization of the root canal morphology using a chitosan-based endo-radiopaque solution
Shashirekha Govind, Amit Jena, Satabdi Pattanaik, Mahaprasad Anarasi, Satyajit Mohapatra, Vinay Shivagange
Restor Dent Endod. 2021;46(3):e33. doi: 10.5395/rde.2021.46.e33.
Reference
-
1. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999; 284:1318–1322. PMID: 10334980.
Article2. Siqueira JF Jr, Paiva SS, Rôças IN. Reduction in the cultivable bacterial populations in infected root canals by a chlorhexidine-based antimicrobial protocol. J Endod. 2007; 33:541–547. PMID: 17437868.
Article3. Pascon FM, Kantovitz KR, Sacramento PA, Nobredos-Santos M, Puppin-Rontani RM. Effect of sodium hypochlorite on dentin mechanical properties. A review. J Dent. 2009; 37:903–908. PMID: 19665276.4. Del Carpio-Perochena AE, Bramante CM, Duarte MA, Cavenago BC, Villas-Boas MH, Graeff MS, Bernardineli N, de Andrade FB, Ordinola-Zapata R. Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J Endod. 2011; 37:1134–1138. PMID: 21763908.
Article5. Zhang K, Kim YK, Cadenaro M, Bryan TE, Sidow SJ, Loushine RJ, Ling JQ, Pashley DH, Tay FR. Effects of different exposure times and concentrations of sodium hypochlorite/ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. J Endod. 2010; 36:105–109. PMID: 20003945.
Article6. Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002; 28:17–19. PMID: 11806642.
Article7. Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:658–666. PMID: 12464887.
Article8. Violich DR, Chandler NP. The smear layer in endodontics - a review. Int Endod J. 2010; 43:2–15. PMID: 20002799.
Article9. Garberoglio R, Becce C. Smear layer removal by root canal irrigants. A comparative scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1994; 78:359–367. PMID: 7970599.10. Kishen A, Sum CP, Mathew S, Lim CT. Influence of irrigation regimens on the adherence of Enterococcus faecalis to root canal dentin. J Endod. 2008; 34:850–854. PMID: 18570994.
Article11. Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, Dhawan S. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004; 274:1–33. PMID: 15072779.
Article12. Xu Z, Neoh KG, Lin CC, Kishen A. Biomimetic deposition of calcium phosphate minerals on the surface of partially demineralized dentin modified with phosphorylated chitosan. J Biomed Mater Res B Appl Biomater. 2011; 98:150–159. PMID: 21538842.13. Shrestha A, Friedman S, Kishen A. Photodynamically crosslinked and chitosan-incorporated dentin collagen. J Dent Res. 2011; 90:1346–1351. PMID: 21911787.
Article14. No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002; 74:65–72. PMID: 11929171.
Article15. Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod. 2008; 34:1515–1520. PMID: 19026885.
Article16. Silva PV, Guedes DF, Nakadi FV, Pécora JD, Cruz-Filho AM. Chitosan: a new solution for removal of smear layer after root canal instrumentation. Int Endod J. 2013; 46:332–338. PMID: 22970844.
Article17. Calamari SE, Bojanich MA, Barembaum SR, Berdicevski N, Azcurra AI. Antifungal and post-antifungal effects of chlorhexidine, fluconazole, chitosan and its combinations on Candida albicans. Med Oral Patol Oral Cir Bucal. 2011; 16:e23–e28. PMID: 20711160.
Article18. Magura ME, Kafrawy AH, Brown CE Jr, Newton CW. Human saliva coronal microleakage in obturated root canals: an in vitro study. J Endod. 1991; 17:324–331. PMID: 1779218.19. Silva PV, Guedes DF, Pécora JD, da Cruz-Filho AM. Time-dependent effects of chitosan on dentin structures. Braz Dent J. 2012; 23:357–361. PMID: 23207849.
Article20. De Moura MR, Aouada FA, Avena-Bustillos RJ, McHugh TH, Krochta JM, Mattoso LHC. Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. J Food Eng. 2009; 92:448–453.
Article21. Zandim DL, Corrêa FO, Rossa Júnior C, Sampaio JE. In vitro evaluation of the effect of natural orange juices on dentin morphology. Braz Oral Res. 2008; 22:176–183. PMID: 18622489.22. Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000; 146:2395–2407. PMID: 11021916.
Article23. Chávez de Paz LE. Image analysis software based on color segmentation for characterization of viability and physiological activity of biofilms. Appl Environ Microbiol. 2009; 75:1734–1739. PMID: 19139239.
Article24. Wegehaupt F, Gries D, Wiegand A, Attin T. Is bovine dentin an appropriate substitute for human dentin in erosion/abrasion tests? J Oral Rehabil. 2008; 35:390–394. PMID: 18405276.25. Whitehead KA, Rogers D, Colligon J, Wright C, Verran J. Use of the atomic force microscope to determine the effect of substratum surface topography on the ease of bacterial removal. Colloids Surf B Biointerfaces. 2006; 51:44–53. PMID: 16822658.
Article26. Lopes MB, Sinhoreti MA, Gonini Júnior A, Consani S, McCabe JF. Comparative study of tubular diameter and quantity for human and bovine dentin at different depths. Braz Dent J. 2009; 20:279–283. PMID: 20069249.
Article27. Del Carpio-Perochena A, Bramante CM, Hungaro Duarte MA, de Andrade FB, Cavenago BC, Villas-Bôas MH, Ordinola-Zapata R, Amoroso-Silva P. Application of laser scanning microscopy for the analysis of oral biofilm dissolution by different endodontic irrigants. Dent Res J (Isfahan). 2014; 11:442–447. PMID: 25225556.28. Ordinola-Zapata R, Bramante CM, Cavenago B, Graeff MS, Gomes de Moraes I, Marciano M, Duarte MA. Antimicrobial effect of endodontic solutions used as final irrigants on a dentin biofilm model. Int Endod J. 2012; 45:162–168. PMID: 21985189.29. Inoue K, Yoshizuka K, Ohto K. Adsorptive separation of some metal ions by complexing agent types of chemically modified chitosan. Anal Chim Acta. 1999; 388:209–218.
Article30. Bassi R, Prasher SO, Simpson BK. Effects of organic acids on the adsorption of heavy metal ions by chitosan flakes. J Environ Sci Health. 1999; 34:289–294.
Article31. Blair HS, Ho TC. Studies in the adsorption and diffusion of ions in chitosan. J Chem Technol Biotechnol. 1981; 31:6–10.
Article32. Vold IMN, Vårum KM, Guibal E, Smidsrød O. Binding of ions to chitosan-selectivity studies. Carbohydr Polym. 2003; 54:471–477.
Article33. Pimenta JA, Zaparolli D, Pécora JD, Cruz-Filho AM. Chitosan: effect of a new chelating agent on the microhardness of root dentin. Braz Dent J. 2012; 23:212–217. PMID: 22814688.
Article34. Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987; 41:435–464. PMID: 3318676.
Article35. Wang QQ, Zhang CF, Chu CH, Zhu XF. Prevalence of Enterococcus faecalis in saliva and filled root canals of teeth associated with apical periodontitis. Int J Oral Sci. 2012; 4:19–23. PMID: 22422085.
Article36. Saunders WP, Saunders EM. Coronal leakage as a cause of failure in root-canal therapy: a review. Endod Dent Traumatol. 1994; 10:105–108. PMID: 7995237.
Article37. Hasan NA, Young BA, Minard-Smith AT, Saeed K, Li H, Heizer EM, McMillan NJ, Isom R, Abdullah AS, Bornman DM, Faith SA, Choi SY, Dickens ML, Cebula TA, Colwell RR. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS One. 2014; 9:e97699. PMID: 24846174.
Article38. Madison S, Swanson K, Chiles SA. An evaluation of coronal microleakage in endodontically treated teeth. Part II. Sealer types. J Endod. 1987; 13:109–112. PMID: 3471832.
Article39. Madison S, Wilcox LR. An evaluation of coronal microleakage in endodontically treated teeth. Part III. In vivo study. J Endod. 1988; 14:455–458. PMID: 3273315.40. Gray GW, Wilkinson SG. The effect of ethylenediaminetetra-acetic acid on cell walls of some gram-negative bacteria. J Gen Microbiol. 1965; 39:385–399. PMID: 4955831.41. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003; 4:1457–1465. PMID: 14606868.
Article42. Young DH, Köhle H, Kauss H. Effect of chitosan on membrane-permeability of suspension-cultured glycine-max and phaseolus-vulgaris cells. Plant Physiol. 1982; 70:1449–1454. PMID: 16662696.43. Persadmehr A, Torneck CD, Cvitkovitch DG, Pinto V, Talior I, Kazembe M, Shrestha S, McCulloch CA, Kishen A. Bioactive chitosan nanoparticles and photodynamic therapy inhibit collagen degradation in vitro. J Endod. 2014; 40:703–709. PMID: 24767568.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chelating and antibacterial properties of chitosan nanoparticles on dentin
- Analysis of the shelf life of chitosan stored in different types of packaging, using colorimetry and dentin microhardness
- Antibacterial Effect on
Enterococcus Faecalis and Physical Properties of Chitosan Added Calcium Hydroxide Canal Filling Material - Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin
- A Comparison Study of Radiostrontium Chelation with Chitin, Chitosan, EDTA and DTPA