J Breast Cancer.
2014 Dec;17(4):344-349. 10.4048/jbc.2014.17.4.344.
A Phase II Trial of Neoadjuvant Chemotherapy with Genexol(R) (Paclitaxel) and Epirubicin for Locally Advanced Breast Cancer
- Affiliations
-
- 1Department of Surgery, Chungnam National University, Daejoen, Korea. kimjr@cnu.ac.kr
- 2Department of Surgery, Wonkwang University School of Medicine, Iksan, Korea.
- 3Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea.
- 4Department of Surgery, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 5Department of Surgery, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- KMID: 2176125
- DOI: http://doi.org/10.4048/jbc.2014.17.4.344
Abstract
- PURPOSE
Neoadjuvant chemotherapy (NC) is yet to be established as the definitive treatment regimen for locally advanced breast cancer (LABC). The aim of this study was to determine the efficacy and toxicity of NC with epirubicin and paclitaxel.
METHODS
Between March 2007 and January 2009, 50 patients with LABC were enrolled in an open-label, phase II, multicenter study carried out at five distinct institutions. All patients were scheduled to receive four cycles of 60 mg/m2 epirubicin and 175 mg/m2 paclitaxel every 3 weeks, preoperatively, unless they developed profound side effects or disease progression. After curative surgery, two additional cycles of chemotherapy were administered to patients who had shown a positive response to NC.
RESULTS
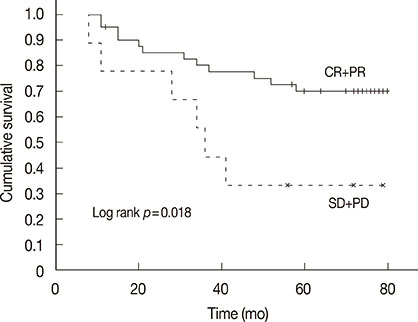
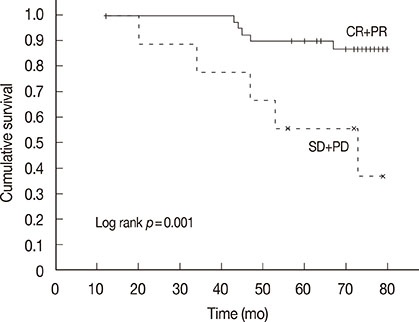
In all, 196 cycles of chemotherapy were administered preoperatively; 47 of the 50 patients (94%) underwent all four cycles of designated treatment. Complete disappearance of invasive foci of the primary tumor, and negative axillary lymph nodes were confirmed in eight patients (16.0%), post operation. The cumulative 5-year disease-free survival rate was 70.0% for patients with complete remission (CR) and partial remission (PR), and 33.3% for patients with stable disease (SD) and progressive disease (PD) (p=0.018). The cumulative 5-year overall survival was 90.0% for patients who achieved CR and PR and 55.6% for patients who had SD and PD (p=0.001). Neutropenia (42.0%) was the most common grade 3/4 toxicity. However, none of the toxicities resulted in cessation of the treatment.
CONCLUSION
The encouraging pathologic response observed in the patients treated with epirubicin plus paclitaxel NC in this study suggests that epirubicin could be a substitute for doxorubicin, which is the most cardiotoxic agent.
Keyword
MeSH Terms
Figure
Reference
-
1. Heys SD, Sarkar T, Hutcheon AW. Primary docetaxel chemotherapy in patients with breast cancer: impact on response and survival. Breast Cancer Res Treat. 2005; 90:169–185.
Article2. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998; 16:2672–2685.
Article3. Buzdar AU. Preoperative chemotherapy treatment of breast cancer: a review. Cancer. 2007; 110:2394–2407.
Article4. Hortobagyi GN, Holmes FA. Optimal dosing of paclitaxel and doxorubicin in metastatic breast cancer. Semin Oncol. 1997; 24:1 Suppl 3. S4–S7.5. Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicinbased neoadjuvant chemotherapy. J Clin Oncol. 1999; 17:460–469.
Article6. Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002; 20:1456–1466.
Article7. Diéras V, Fumoleau P, Romieu G, Tubiana-Hulin M, Namer M, Mauriac L, et al. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol. 2004; 22:4958–4965.
Article8. Gianni L, Munzone E, Capri G, Fulfaro F, Tarenzi E, Villani F, et al. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol. 1995; 13:2688–2699.
Article9. Conte PF, Baldini E, Gennari A, Michelotti A, Salvadori B, Tibaldi C, et al. Dose-finding study and pharmacokinetics of epirubicin and paclitaxel over 3 hours: a regimen with high activity and low cardiotoxicity in advanced breast cancer. J Clin Oncol. 1997; 15:2510–2517.
Article10. Cersosimo RJ, Hong WK. Epirubicin: a review of the pharmacology, clinical activity, and adverse effects of an adriamycin analogue. J Clin Oncol. 1986; 4:425–439.
Article11. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.
Article12. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001; 19:4224–4237.
Article13. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997; 15:2483–2493.
Article14. Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003; 21:4165–4174.
Article15. Dombernowsky P, Boesgaard M, Andersen E, Jensen BV. Doxorubicin plus paclitaxel in advanced breast cancer. Semin Oncol. 1997; 24:5 Suppl 17. S17.16. Coukell AJ, Faulds D. Epirubicin: an updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of breast cancer. Drugs. 1997; 53:453–482.17. Danesi R, Conte PF, Del Tacca M. Pharmacokinetic optimisation of treatment schedules for anthracyclines and paclitaxel in patients with cancer. Clin Pharmacokinet. 1999; 37:195–211.
Article18. Jain KK, Casper ES, Geller NL, Hakes TB, Kaufman RJ, Currie V, et al. A prospective randomized comparison of epirubicin and doxorubicin in patients with advanced breast cancer. J Clin Oncol. 1985; 3:818–826.
Article19. Gogas H, Papadimitriou C, Kalofonos HP, Bafaloukos D, Fountzilas G, Tsavdaridis D, et al. Neoadjuvant chemotherapy with a combination of pegylated liposomal doxorubicin (Caelyx) and paclitaxel in locally advanced breast cancer: a phase II study by the Hellenic Cooperative Oncology Group. Ann Oncol. 2002; 13:1737–1742.
Article20. Bellino R, Cortese P, Danese S, De Sanctis C, Durando A, Genta F, et al. Epidoxorubicin and paclitaxel as primary chemotherapy for T > 3 cm and T4 breast cancer patients. Anticancer Res. 2000; 20(6C):4825–4828.21. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.
Article22. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–785.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical outcome and predictive factors for docetaxel and epirubicin neoadjuvant chemotherapy of locally advanced breast cancer
- Phase II Study of Cisplatin, Ifosfamide . Paclitaxel (CIP) as Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Carcinoma
- Preoperative Chemotherapy in Advanced Stomach Cancer (Pros)
- Cost-Effectiveness of Genexol-PM for Treating Metastatic Breast Cancer
- Phase II Study of CP (Cisplatin/Paclitaxel) as Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Carcinoma