Diabetes Metab J.
2015 Aug;39(4):335-341. 10.4093/dmj.2015.39.4.335.
Effects of 6-Month Sitagliptin Treatment on Insulin and Glucagon Responses in Korean Patients with Type 2 Diabetes Mellitus
- Affiliations
-
- 1Divison of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. drhopper@catholic.ac.kr
- KMID: 2174030
- DOI: http://doi.org/10.4093/dmj.2015.39.4.335
Abstract
- BACKGROUND
This study aimed to evaluate the effect of sitagliptin, an oral dipeptidyl peptidase-4 inhibitor, on insulin secretion and glucagon suppression in Korean subjects with type 2 diabetes mellitus.
METHODS
Twenty-four subjects underwent a 75-g oral glucose tolerance test (OGTT) before and after 6 months of sitagliptin treatment. Sitagliptin, insulin, and sulfonylurea were withdrawn for 3 days before OGTT to eliminate any acute effects on beta-cell insulin or alpha-cell glucagon secretion. Venous samples were drawn five times during each OGTT to measure plasma glucose, insulin, and glucagon. Indices on insulin secretion and resistance were calculated.
RESULTS
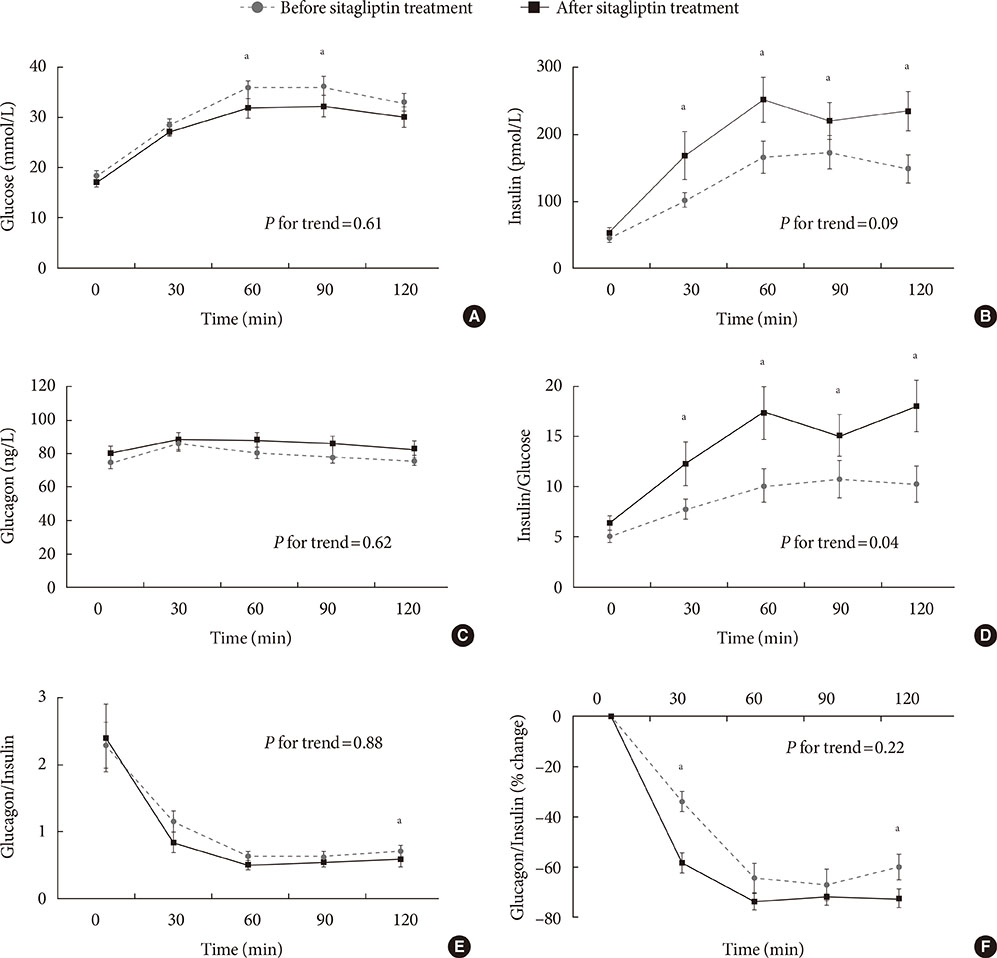
Early phase insulin secretion, measured by the insulinogenic index significantly increased after 6 months of sitagliptin treatment, especially in the higher baseline body mass index group and higher baseline glycosylated hemoglobin (HbA1c) group. There were no significant differences in the insulin resistance indices before and after sitagliptin treatment. Although no significant differences were observed in the absolute levels of glucagon and the glucagon-to-insulin ratio, there was a significant reduction in the percentile change of glucagon-to-insulin ratio at 30- and 120-minute during the OGTT.
CONCLUSION
Although the HbA1c level did not decrease significantly after 6 months of sitagliptin treatment, an increase in insulin secretion and reduction in early phase postprandial plasma glucagon-to-insulin ratio excursion was confirmed in Korean subjects with type 2 diabetes.
MeSH Terms
Figure
Reference
-
1. Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001; 9:381–387.2. He Q, Horlick M, Thornton J, Wang J, Pierson RN Jr, Heshka S, Gallagher D. Sex and race differences in fat distribution among Asian, African-American, and Caucasian prepubertal children. J Clin Endocrinol Metab. 2002; 87:2164–2170.3. Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004; 66:Suppl 1. S37–S43.4. Chen KW, Boyko EJ, Bergstrom RW, Leonetti DL, Newell-Morris L, Wahl PW, Fujimoto WY. Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM. 5-Year follow-up of initially nondiabetic Japanese-American men. Diabetes Care. 1995; 18:747–753.5. Ferrannini E. The stunned beta cell: a brief history. Cell Metab. 2010; 11:349–352.6. Vilsboll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004; 47:357–366.7. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013; 56:696–708.8. Dhillon S. Sitagliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2010; 70:489–512.9. Matsumoto S, Yamazaki M, Kadono M, Iwase H, Kobayashi K, Okada H, Fukui M, Hasegawa G, Nakamura N. Effects of liraglutide on postprandial insulin and glucagon responses in Japanese patients with type 2 diabetes. J Clin Biochem Nutr. 2013; 53:68–72.10. Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, Vilsboll T. Preserved inhibitory potency of GLP-1 on glucagon secretion in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009; 94:4679–4687.11. Eto T, Inoue S, Kadowaki T. Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2012; 14:1040–1046.12. Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, Lasseter KC, Kipnes MS, Snyder K, Hilliard D, Tanen M, Cilissen C, De Smet M, de Lepeleire I, Van Dyck K, Wang AQ, Zeng W, Davies MJ, Tanaka W, Holst JJ, Deacon CF, Gottesdiener KM, Wagner JA. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006; 91:4612–4619.13. DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008; 24:2943–2952.14. Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006; 29:2632–2637.15. Gudipaty L, Rosenfeld NK, Fuller CS, Gallop R, Schutta MH, Rickels MR. Effect of exenatide, sitagliptin, or glimepiride on beta-cell secretory capacity in early type 2 diabetes. Diabetes Care. 2014; 37:2451–2458.16. Kim SA, Shim WH, Lee EH, Lee YM, Beom SH, Kim ES, Yoo JS, Nam JS, Cho MH, Park JS, Ahn CW, Kim KR. Predictive clinical parameters for the therapeutic efficacy of sitagliptin in korean type 2 diabetes mellitus. Diabetes Metab J. 2011; 35:159–165.17. Kim WJ, Park CY, Jeong EH, Seo JY, Seol JS, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW, Kim SW. Retrospective analysis on the efficacy, safety and treatment failure group of sitagliptin for mean 10-month duration. Diabetes Metab J. 2011; 35:290–297.18. Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011; 13:7–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Sitagliptin on Insulin and Glucagon Levels in Type 2 Diabetes Mellitus
- Glucagon-Like Peptide-1 (GLP-1) Agonist
- Pancreatic alpha-Cell Dysfunction in Type 2 Diabetes: Old Kids on the Block
- New Potential Targets of Glucagon-Like Peptide 1 Receptor Agonists in Pancreatic β-Cells and Hepatocytes
- Clinical Application of Glucagon-Like Peptide-1 Receptor Agonists