Ann Dermatol.
2013 May;25(2):218-222. 10.5021/ad.2013.25.2.218.
Efficacy and Safety of Pueraria lobata Extract in Gray Hair Prevention: A Randomized, Double-Blind, Placebo-Controlled Study
- Affiliations
-
- 1Department of Dermatology, Seoul National University College of Medicine, Seoul, Korea. oskwon@snu.ac.kr
- 2Department of Dermatology, Yanbian University Hospital, Jilin, China.
- 3Advanced Hair Research Laboratory, AMOREPACIFIC Corp. R&D Center, Yongin, Korea.
- KMID: 2171792
- DOI: http://doi.org/10.5021/ad.2013.25.2.218
Abstract
- BACKGROUND
Graying of hair-a sign of aging-raises cosmetic concerns. Individuals with gray hair often look older than others their age; therefore, some dye their hair for aesthetic purposes. However, hair colorants can induce many problems including skin irritation, allergic reaction and hair-breakage.
OBJECTIVE
This randomized, double-blind clinical trial was performed in order to examine the effects of APHG-1001, a compound including an extract from Pueraria lobata, on graying hair.
METHODS
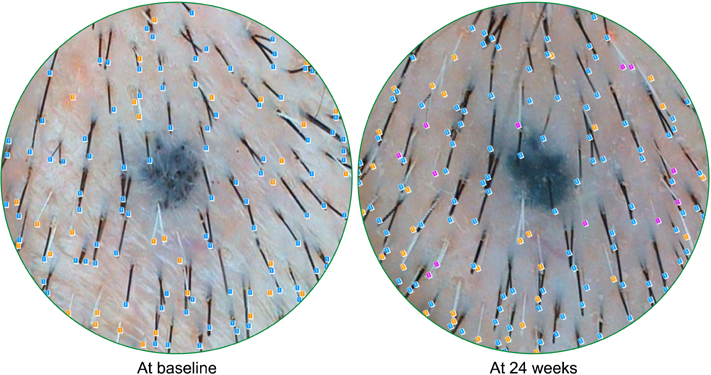
A total of 44 female subjects were randomly treated with either APHG-1001 or placebo twice daily for 24 weeks. Using the phototrichogram analysis, a count of newly developed gray hair was estimated. Investigator assessment and subject self-assessment were also performed in order to evaluate the efficacy of the compound.
RESULTS
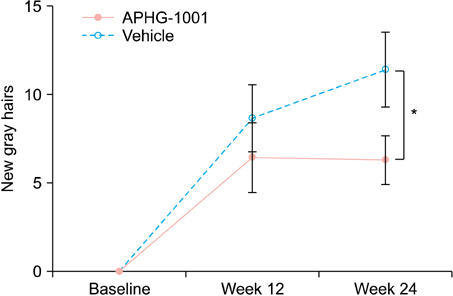
The mean number of newly developed gray hair at 24 weeks was 6.3/cm2 in the APHG-1001 group and 11.4/cm2 in the placebo group; the difference was statistically significant (p<0.05). However, the investigator assessment and subject self-assessment did not show any significant change in the gross appearance of hair grayness by the end of the study. No severe adverse events in either group were observed. Moreover, the incidence of adverse events did not differ between the groups.
CONCLUSION
This clinical trial revealed that APHG-1001, which contains an extract of P. lobata, could prevent the development of new gray hair without any remarkable adverse effects. Thus, it can be considered as a viable treatment option for the prevention of gray hair.
Keyword
MeSH Terms
Figure
Reference
-
1. Trüeb RM. Aging of hair. J Cosmet Dermatol. 2005. 4:60–72.
Article2. Tobin DJ, Paus R. Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001. 36:29–54.
Article3. Keogh EV, Walsh RJ. Rate of greying of human hair. Nature. 1965. 207:877–878.
Article4. Lapeere H, Boone B, Schepper SD, Verhaeghe E, Ongenae K, Geel NV, et al. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Hypomelanoses and hypermelanoses. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill Medical;622–640.5. Cline DJ. Changes in hair color. Dermatol Clin. 1988. 6:295–303.
Article6. Jo SJ, Paik SH, Choi JW, Lee JH, Cho S, Kim KH, et al. Hair graying pattern depends on gender, onset age and smoking habits. Acta Derm Venereol. 2012. 92:160–161.
Article7. Bulpitt CJ, Markowe HL, Shipley MJ. Why do some people look older than they should? Postgrad Med J. 2001. 77:578–581.
Article8. Jo SJ, Shin HS, Paik SH, Choi JW, Lee JH, Cho S, et al. The pattern of hair dyeing in Koreans with gray hair. Ann Dermatol. In press 2013.
Article9. Thyssen JP, White JM. European Society of Contact Dermatitis. Epidemiological data on consumer allergy to p-phenylenediamine. Contact Dermatitis. 2008. 59:327–343.10. Zhang YP, Shi SY, Xiong X, Chen XQ, Peng MJ. Comparative evaluation of three methods based on high-performance liquid chromatography analysis combined with a 2,2'-diphenyl-1-picrylhydrazyl assayfor the rapid screening of antioxidants from Pueraria lobata flowers. Anal Bioanal Chem. 2012. 402:2965–2976.
Article11. Jiang RW, Lau KM, Lam HM, Yam WS, Leung LK, Choi KL, et al. A comparative study on aqueous root extracts of Pueraria thomsonii and Pueraria lobata by antioxidant assay and HPLC fingerprint analysis. J Ethnopharmacol. 2005. 96:133–138.
Article12. Cherdshewasart W, Sutjit W. Correlation of antioxidant activity and major isoflavonoid contents of the phytoestrogenrich Pueraria mirifica and Pueraria lobata tubers. Phytomedicine. 2008. 15:38–43.
Article13. Wood JM, Decker H, Hartmann H, Chavan B, Rokos H, Spencer JD, et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009. 23:2065–2075.
Article14. Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF, et al. Towards a "free radical theory of graying": melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006. 20:1567–1569.
Article15. Commo S, Gaillard O, Bernard BA. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br J Dermatol. 2004. 150:435–443.
Article16. Bebrevska L, Foubert K, Hermans N, Chatterjee S, Van Marck E, De Meyer G, et al. In vivo antioxidative activity of a quantified Pueraria lobata root extract. J Ethnopharmacol. 2010. 127:112–117.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy and Safety of the Topical Application of Poly-Gamma Glutamic Acid Hydrogel Nanoparticle-Based Fermented Extract of a Medicinal Plant Solution for Androgenetic Alopecia: A Randomized Double-Blinded Placebo-Controlled Clinical Trial
- The Efficacy and Safety of AP-FHG0604T on Female Pattern Hair Loss: A Randomized Double-blind Placebo-controlled Clinical Trial
- Efficacy and safety of mirikizumab as induction and maintenance therapy for Japanese patients with moderately to severely active ulcerative colitis: a subgroup analysis of the global phase 3 LUCENT-1 and LUCENT-2 studies
- Prophylactic efficacy of probiotics on travelers' diarrhea: an adaptive meta-analysis of randomized controlled trials
- The efficacy and safety of Dendropanax morbifera leaf extract on the metabolic syndrome: a 12-week, placebo controlled, double blind, and randomized controlled trial