Ann Clin Microbiol.
2013 Mar;16(1):1-7. 10.5145/ACM.2013.16.1.1.
Anti-Tuberculosis Drug Resistant Rates in Mycobacterium tuberculosis Isolated from Respiratory Specimens: A Multicenter Study in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. yjpk@catholic.ac.kr
- 2Department of Laboratory Medicine, Samsung Medical Center, Sungkyunkwan University, School of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 4Department of Laboratory Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 5Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2169595
- DOI: http://doi.org/10.5145/ACM.2013.16.1.1
Abstract
- BACKGROUND
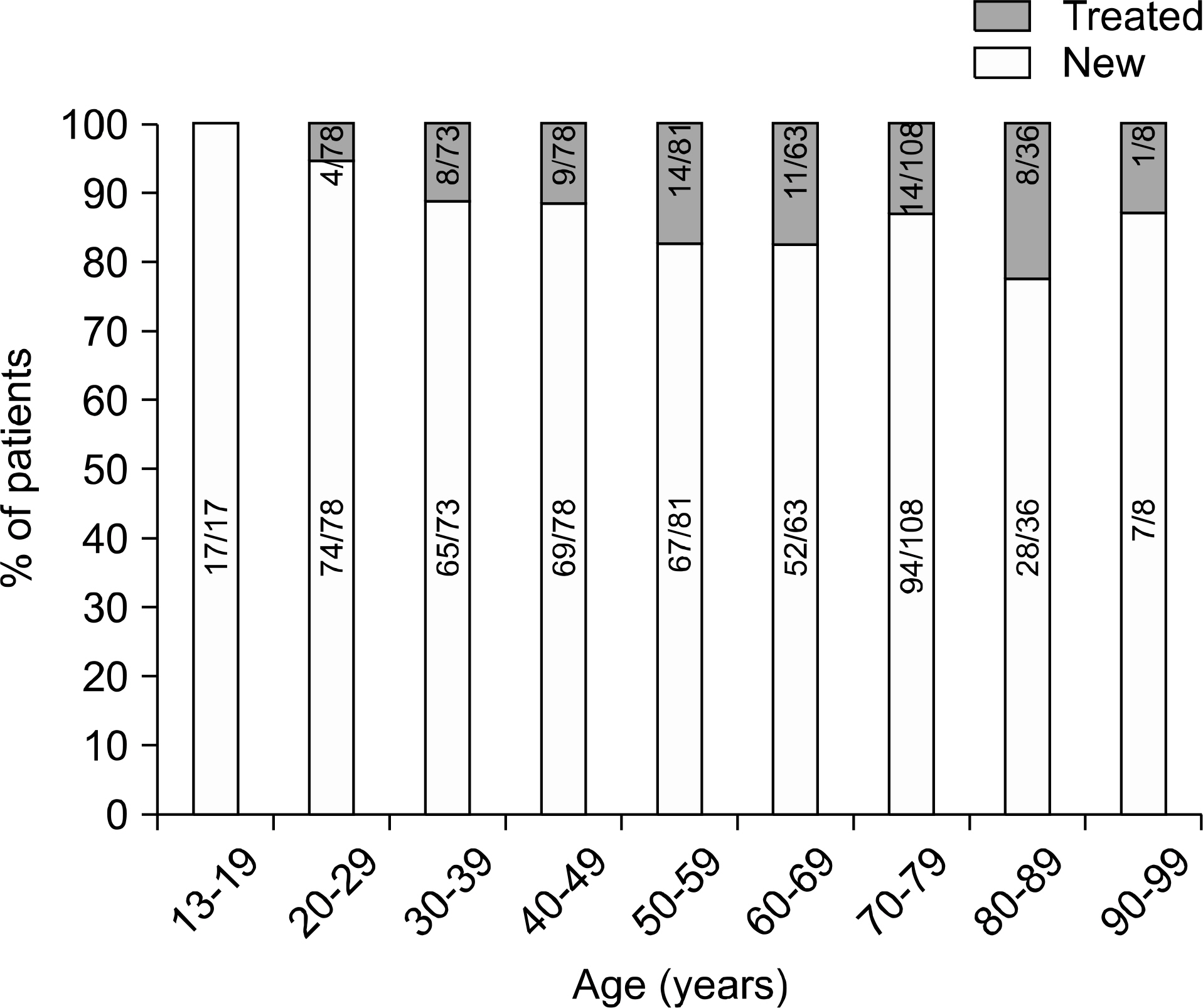
We analyzed the prevalence of anti-tuberculosis drug resistance in Mycobacterium tuberculosis isolates from respiratory specimens of patients with newly diagnosed and previously treated tuberculosis.
METHODS
From February 2010 to July 2010, a total of 542 M. tuberculosis clinical isolates were collected from pulmonary tuberculosis patients in six university hospitals distributed throughout Korea. We analyzed the results of anti-tuberculosis drug resistance tests according to treatment history and geographic location.
RESULTS
Among the 542 isolates, 473 (87.3%) were from newly diagnosed cases and 69 (12.7%) were from previously treated cases. The rates of multi-drug resistance (MDR), fluoroquinolone (ofloxacin, levofloxacin, and moxifloxacin) resistance, and extensive drug resistance (XDR) were 3.8%, 1.1-1.5%, and 0%, respectively, in new cases, and 21.7%, 13.0-17.4%, and 4.3%, respectively, in previously treated cases. In the previously treated cases, the proportions of XDR-TB in MDR-TB were 20% (3/15). The resistance rates were variable according to geographic location.
CONCLUSION
As the anti-tuberculosis drug resistance rates are much higher in newly diagnosed cases than in previously treated patients, efforts should be made to ensure that tuberculosis treatment is successful. In addition, before the selection of an anti-tuberculosis drug treatment for previously treated patients, the susceptibility test results, including to fluoroquinolone, should be verified.
Keyword
MeSH Terms
Figure
Reference
-
1.Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, Reniero. $$$$.2.World Health Organization. Guidelines for the Programmatic Management of Drug Resistant Tuberculosis: Emergency Update 2008. Geneva, Switzerland: World Health Organization;2008.3.Korea Centers for Disease Control and Prevention. Annual Report on the Notified Tuberculosis Patients in Korea 2009. Seoul: Korea Centers for Disease Control and Prevention;2010.4.Wright A., Zignol M., Van Deun A., Falzon D., Gerdes SR., Feldman K, et al. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuber-culosis Drug Resistance Surveillance. Lancet. 2009. 373:1861–73.
Article5.Bloch AB., Cauthen GM., Onorato IM., Dansbury KG., Kelly GD., Driver CR, et al. Nationwide survey of drug-resistant tuberculosis in the United States. JAMA. 1994. 271:665–71.
Article6.Joint Committee for the Development of Korean Guidelines for Tuberculosis. Korean Guidelines for Tuberculosis. 1st ed.Seoul: Korea Centers for Disease Control and Prevention;2011. p. 34–6.7.Jeong SH., Lee DD., Choi JC., Kim S., Shin JH., Jeong J, et al. Multicenter study on cost effectiveness of antituberculosis drug susceptibility test. Infect Chemother. 2005. 37:16–21.8.Kim SJ., Bai GH., Hong YP. Drug-resistant tuberculosis in Korea, 1994. Int J Tuberc Lung Dis. 1997. 1:302–8.9.Bai GH., Park YK., Choi YW., Bai JI., Kim HJ., Chang CL, et al. Trend of antituberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007. 11:571–6.10.Kim BJ., Lee IH., Lee DH., Bai GH., Kong SJ., Lee SH, et al. The current status of multidrug-resistant tuberculosis in Korea. Tuberc Respir Dis. 2006. 60:404–11.
Article11.Chol CJ., Lim SY., Suh GY., Chung MP., Kim H., Kwon OJ, et al. Drug resistance rates of Mycobacterium tuberculosis at a private referral center in Korea. J Korean Med Sci. 2007. 22:677–81.12.KOSIS. Population of working age (15 to 64) by sex and age group. Korean Statistical Information Service Web Site on Online Publication.http://kosis.kr/wnsearch/totalSearch.jsp/[Online. (last visited on 11 September 2012).13.Lee SJ., Ahn YM., Kim HJ. Drug resistance of Mycobacterium tuberculosis in children. Korean J Pediatr. 2009. 52:61–7.14.14.Centers for Disease Control and Prevention (CDC). Emergence of Mycobacterium tuberculosis with extensive resistance to second- line drugs-worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006. 55:301–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug Resistance of Mycobacterium tuberculosis isolated from Patients discovered in the Third National Tuberculosis Prevalence Survey in 1975 in Korea
- Rifampin-resistant Relapsed Tuberculosis Confirmed by Molecular Technique
- Phagocytosis of Drug-Resistant Mycobacterium Tuberculosis by Peripheral Blood Monocytes
- Crossresistance between Tuberactinomycin-N, Capreomycin and Kanamycin of Mycobacterium tuberculosis isolated from patients with pulmonary tuberculosis
- Drug resistance of mycobacterium tuberculosis in Korea