Anat Cell Biol.
2010 Jun;43(2):132-139. 10.5115/acb.2010.43.2.132.
Expression of calponin in periglomerular myofibroblasts of rat kidney with experimental chronic injuries
- Affiliations
-
- 1Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul, Korea. jhcha@catholic.ac.kr
- 2Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2168886
- DOI: http://doi.org/10.5115/acb.2010.43.2.132
Abstract
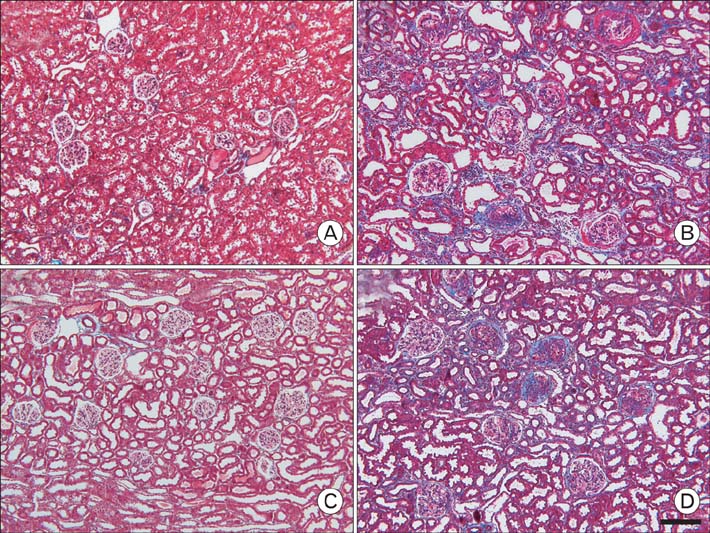
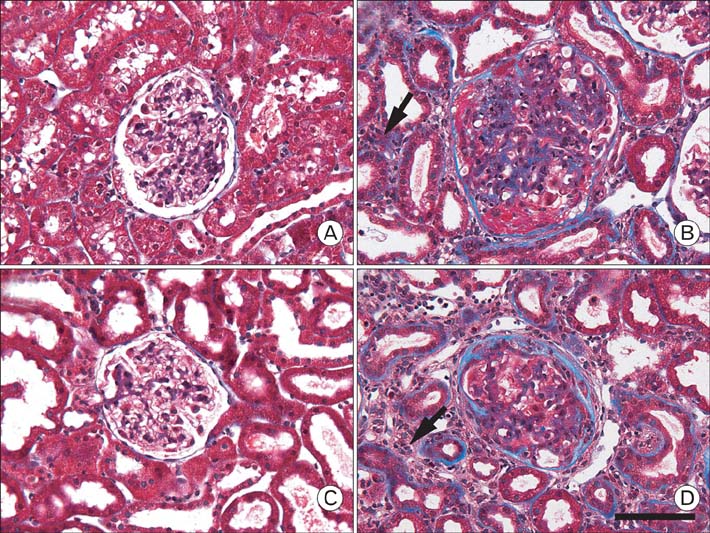
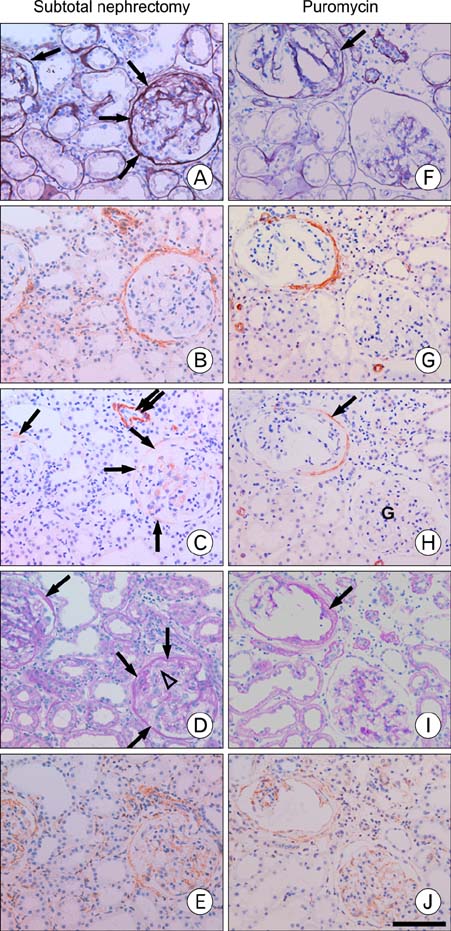
- Our previous research demonstrated that calponin-immunoreactivity was localized in myofibroblasts of the periglomerular region of human kidney specimens obtained at the time of transplantation from organ recipients. In the present study we examined calponin expression in two chronic nephropathy models, puromycin aminonucleoside (PAN) nephropathy and subtotal nephrectomy (SNx), to investigate the role of calponin in chronic renal injury. Male Sprague-Dawley rats were used, and both nephropathy models were established at 1, 2, 4, and 8 weeks after surgery. There were no periglomerular calponin-positive cells in sham, PAN 1 and 2 week, and SNx 1, 2, and 4 week groups. In SNx 8 week and PAN 4 and 8 week groups, only a few glomeruli with periglomerular calponin-reactivity, which covered half or a very small part of the periglomerular space, were observed. All glomeruli with periglomerular calponin-reactivity showed sclerotic changes, especially thickening of parietal epithelial cells (PECs). In conjunction with our previous report, this data represents the first documentation of the expression of calponin in renal myofibroblasts. We suggest that interactions between PECs and calponin-positive myofibroblasts may play a key role in the late stage of glomerulosclerosis.
MeSH Terms
-
Animals
Calcium-Binding Proteins
Epithelial Cells
Humans
Immunohistochemistry
Kidney
Kidney Failure, Chronic
Male
Microfilament Proteins
Myofibroblasts
Nephrectomy
Puromycin Aminonucleoside
Rats
Rats, Sprague-Dawley
Salicylamides
Transplants
Calcium-Binding Proteins
Microfilament Proteins
Puromycin Aminonucleoside
Salicylamides
Figure
Reference
-
1. Alpers CE, Hudkins KL, Floege J, Johnson RJ. Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol. 1994. 5:201–209.2. Ardiles LG, Figueroa CD, Mezzano SA. Renal kallikrein-kinin system damage and salt sensitivity: insights from experimental models. Kidney Int Suppl. 2003. 10. 86:S2–S8.3. Asano T, Niimura F, Pastan I, Fogo AB, Ichikawa I, Matsusaka T. Permanent genetic tagging of podocytes: fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol. 2005. 16:2257–2262.4. Badid C, Desmoulière A, McGregor B, et al. Interstitial alphasmooth muscle actin: A prognostic marker in membranous nephropathy. Clin Nephrol. 1999. 52:210–217.5. Badid C, Mounier N, Costa AM, Desmoulière A. Role of myofibroblasts during normal tissue repair and excessive scarring: Interest of their assessment in nephropathies. Histol Histopathol. 2000. 15:269–280.6. Barbareschi M, Pecciarini L, Cangi MG, et al. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol. 2001. 25:1054–1060.7. Bohle A, Mackensen-Haen S, von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol. 1987. 7:421–433.8. Boucher A, Droz D, Adafer E, Noèl LH. Relationship between the integrity of Bowman's capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest. 1987. 56:526–533.9. Chen W, Chu Y, Zhu D, et al. Perivascular gene transfer of dominant-negative N19RhoA attenuates neointimal formation via inhibition of TGF-beta1-Smad2 signaling in rats after carotid artery balloon injury. Biochem Biophys Res Commun. 2009. 389:217–223.10. Choi JY, Lee SY, Jin DC, et al. Expression of Calponin in Immunoglobulin A Nephropathy: Proteomics and Immunohistochemical Study. 2008. In : Renal week; American Society of Nephrology;[Abstract].11. Cockwell P, Brooks CJ, Adu D, Savage CO. Interleukin-8: a pathogenetic role in antineutrophil cytoplasmic autoantibodyassociated glomerulonephritis. Kidney Int. 1999. 55:852–863.12. Cockwell P, Howie AJ, Adu D, Savage CO. In situ analysis of C-C chemokine mRNA in human glomerulonephritis. Kidney Int. 1998. 54:827–836.13. Essawy M, Soylemzoglu O, Muchaneta-kubara EC, Shortland J, Brown CB, el Nahas AM. Myofibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant. 1997. 12:43–50.14. Ferguson HE, Kulkarni A, Lehmann GM, et al. Electrophilic peroxisome proliferator-activated receptor-γ ligands have potent antifibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol. 2009. 41:722–730.15. Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res. 2000. 48:89–100.16. Goumenos D, Brown CB, Shortland J, El Nahas AM. Myofibroblasts, predictors of progression of mesangial IgA nephropathy? Nephrol Dial Transplant. 1994. 9:1418–1425.17. Goumenos DS, Tsomi K, Iatrou C, et al. Myofibroblasts and the progression of crescentic glomerulonephritis. Nephrol Dial Transplant. 1998. 13:1652–1661.18. Grond J, Weening JJ, van Goor H, Elema JD. Application of puromycin aminonucleoside and adriamycin to induce chronic renal failure in the rat. Contrib Nephrol. 1988. 60:83–93.19. Högemann B, Gillessen A, Böcker W, Rauterberg J, Domschke W. Myofibroblast-like cells produce mRNA for type I and III procollagens in chronic active hepatitis. Scand J Gastroenterol. 1993. 28:591–594.20. Islam AH, Ehara T, Kato H, et al. Calponin h1 expression in renal tumor vessels: correlations with multiple pathological factors of renal cell carcinoma. J Urol. 2004a. 171:1319–1323.21. Islam AH, Ehara T, Kato H, Hayama M, Nishizawa O. Loss of calponin h1 in renal angiomyolipoma correlates with aggressive clinical behavior. Urology. 2004b. 64:468–473.22. Lazard D, Sastret X, Frid MG, Glukhova MA, Thiery JP, Koteliansky VE. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci U S A. 1993. 90:999–1003.23. Nakamura H, Kitazawa K, Honda H, Sugisaki T. Roles of and correlation between alpha-smooth muscle actin, CD44, hyaluronic acid and osteopontin in crescent formation in human glomerulonephritis. Clin Nephrol. 2005. 64:401–411.24. Prasad AP, Savera AT, Gown AM, Zarbo RJ. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999. 123:801–806.25. Roberts IS, Burrows C, Shanks JH, Venning M, Mcwilliam LJ. Interstitial myofibroblasts: Predictors of progression in membranous nephropathy. J Clin Pathol. 1997. 50:123–127.26. Rozenblum GT, Gimona M. Calponins: adaptable modular regulators of the actin cytoskeleton. Int J Biochem Cell Biol. 2008. 40:1990–1995.27. Stahl PJ, Felsen D. Transforming growth factor-beta, basement membrane, and epithelial-mesenchymal transdifferentiation: implications for fibrosis in kidney disease. Am J Pathol. 2001. 159:1187–1192.28. Takahashi K, Hiwada K, Kokubu T. Isolation and characterization of a 34000 Dalton calmodulin- and factin-binding protein from chicken gizzard smooth muscle. Biochem Biophys Res Commun. 1986. 141:20–26.29. Tang WW, Van GY, Qi M. Myofibroblast and alpha1(III) collagen expression in experimental tubulointerstitial nephritis. Kidney Int. 1997. 51:926–931.30. Yanagisawa Y, Takeoka M, Ehara T, Itano N, Miyagawa S, Taniguchi S. Reduction of Calponin h1 expression in human colon cancer blood vessels. Eur J Surg Oncol. 2008. 34:531–537.31. Zhang G, Moorhead PJ, El Nahas AM. Myofibroblasts and the progression of experimental glomerulonephritis. Exp Nephrol. 1995. 3:308–318.32. Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996. 148:527–537.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution and synaptic organization of nitric oxide synthase immunoreactive neurons in the rat olfactory
- Comparison of estrogen receptor-alpha, progesterone receptor and calponin expression in gonadotrophin-releasing hormone agonist-sensitive and -resistant uterine fibroids
- Immunohistochemical Study of Calponin, Smooth Muscle Myosin Heavy Chain, Cytokeratin 34E12, and p53 in Papillary Neoplasm of the Breast
- Experimental evidence that preexisting chronic kidney disease is a risk factor for acute kidney injury
- Development of Experimental Model of Chronic Renal Failure in the Rat