Clin Exp Otorhinolaryngol.
2016 Mar;9(1):14-20. 10.21053/ceo.2016.9.1.14.
The Effect of Dexpanthenol on Ototoxicity Induced by Cisplatin
- Affiliations
-
- 1Department of Otorhinolaryngology, Inonu University Medical Faculty, Malatya, Turkey.
- 2Department of Otorhinolaryngology, Malatya State Hospital, Malatya, Turkey. emrhils@yahoo.com
- 3Department of Pharmacology, Inonu University Medical Faculty, Malatya, Turkey.
- 4Department of Otorhinolaryngology, Sutcu Imam University Medical Faculty, Kahramanmaras, Turkey.
- 5Department of Otorhinolaryngology, Istanbul Medeniyet University Medical Faculty, Istanbul, Turkey.
- 6Department of Physiology, Inonu University Medical Faculty, Malatya, Turkey.
- KMID: 2166276
- DOI: http://doi.org/10.21053/ceo.2016.9.1.14
Abstract
OBJECTIVES
This study was aimed to investigate the protective effects of dexpanthenol (Dxp) on against cisplatin-induced ototoxicity.
METHODS
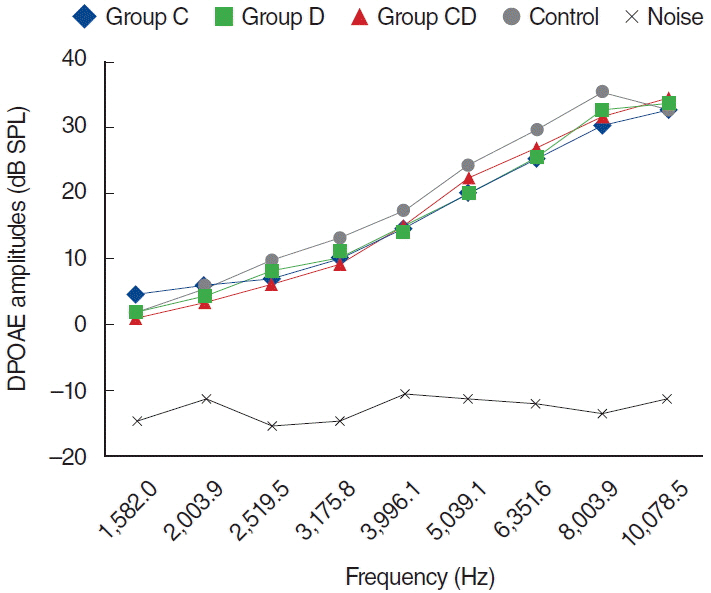
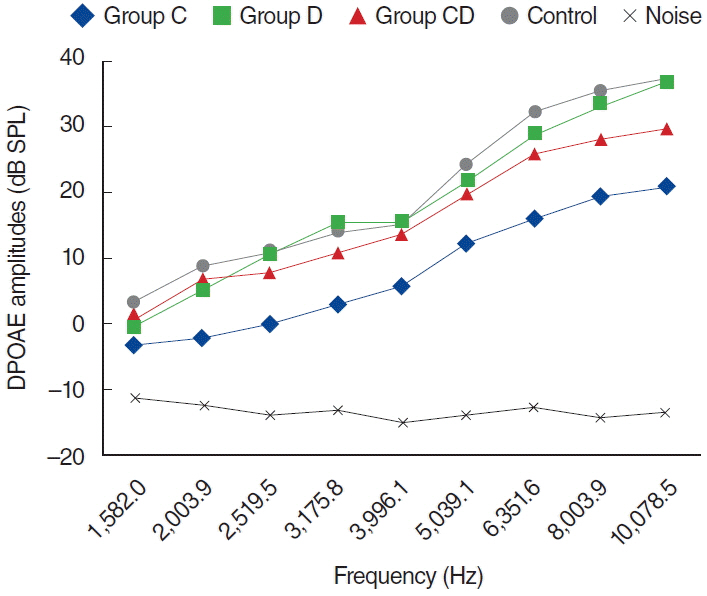
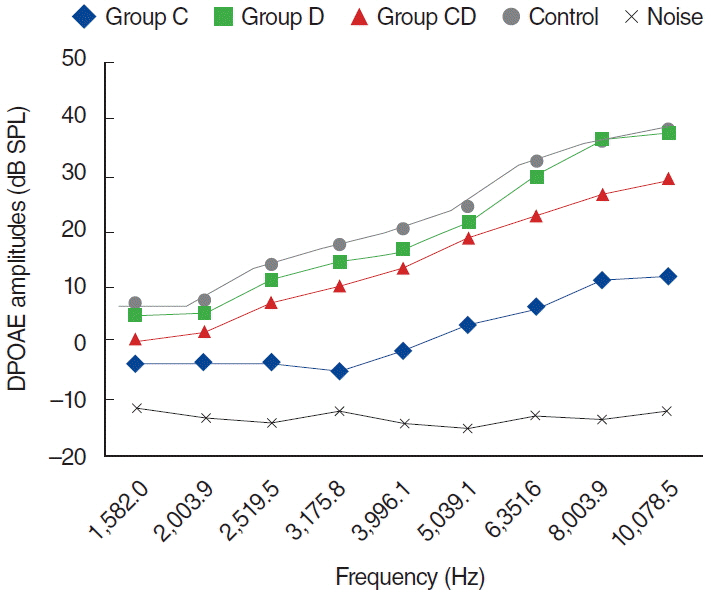
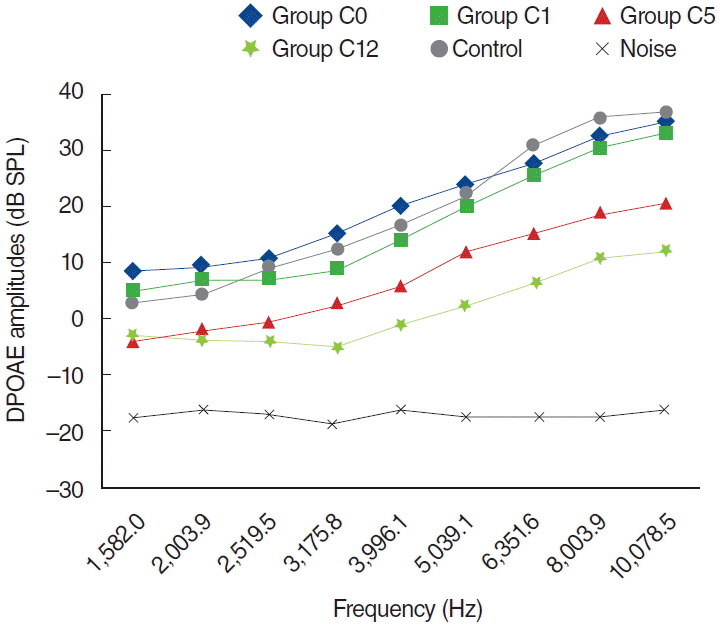
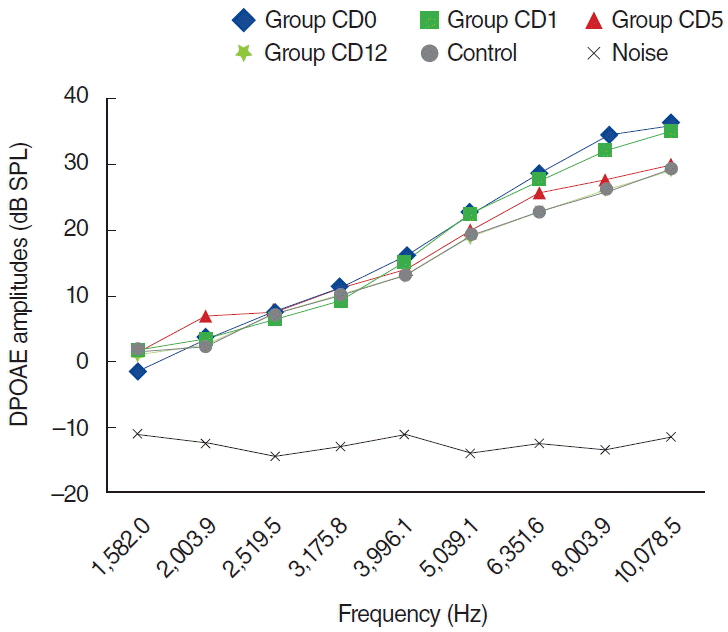
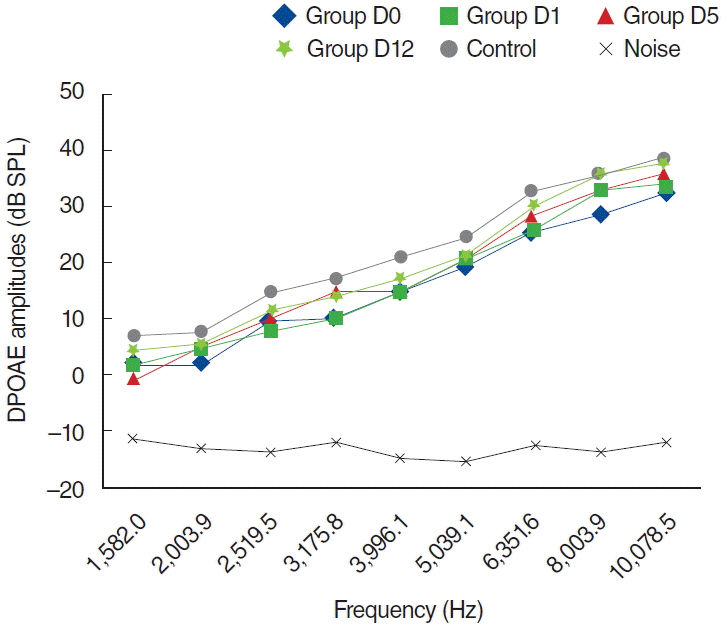
To examine this effect, distortion product otoacoustic emissions (DPOAEs) measurements and serum levels of oxidative and antioxidant status (including malondialdehyde, superoxide dismutase, catalase, glutathione, glutathione peroxidase, total oxidant status, total antioxidant status, and oxidative stress index) were evaluated. Thirty-two adult female Wistar albino rats were randomly divided into 4 equal groups; control (K), cisplatin (C), cisplatin plus Dxp (CD), and Dxp (D). In all groups DPOAEs measurements, between 996 and 10,078 Hz as DPOAEs and input/output functions, were performed on days 0, 1th, 5th, and 12th. Prior to death, the last DPOAEs measurements and blood samples were taken.
RESULTS
In the C group, statistically significant differences were detected at all frequencies between 0 and 5 days and 0 and 12 days measurements (P<0.05). Serum level of oxidant and antioxidant status were detected statistically significantly changed in this group versus K group (P<0.05). Contrary to the C group, in the CD group hearing ability was seen largely preserved at many frequencies and serum levels of all biochemical parameters were shifted toward normal values, similar to the K group. No significant differences were detected in the either D or K group's measurements.
CONCLUSION
According to these results, Dxp may prevent cisplatin-induced ototoxicity.
Keyword
MeSH Terms
Figure
Reference
-
1. Williams CJ, Whitehouse JM. Cis-platinum: a new anticancer agent. Br Med J. 1979; Jun. 1(6179):1689–91.
Article2. Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007; Feb. 33(1):9–23.
Article3. Rossof AH, Slayton RE, Perlia CP. Preliminary clinical experience with cis-diamminedichloroplatinum (II) (NSC 119875, CACP). Cancer. 1972; Dec. 30(6):1451–6.4. Kopelman J, Budnick AS, Sessions RB, Kramer MB, Wong GY. Ototoxicity of high-dose cisplatin by bolus administration in patients with advanced cancers and normal hearing. Laryngoscope. 1988; Aug. 98(8 Pt 1):858–64.
Article5. Kalcioglu MT, Kizilay A, Gulec M, Karatas E, Iraz M, Akyol O, et al. The protective effect of erdosteine against ototoxicity induced by cisplatin in rats. Eur Arch Otorhinolaryngol. 2005; Oct. 262(10):856–63.
Article6. Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F. Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res. 2000; Mar. 28(2):73–80.
Article7. Campbell KC, Rybak LP, Meech RP, Hughes L. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996; Dec. 102(1-2):90–8.
Article8. Campbell KC, Meech RP, Rybak LP, Hughes LF. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol. 2003; Apr. 14(3):144–56.9. Ebner F, Heller A, Rippke F, Tausch I. Topical use of dexpanthenol in skin disorders. Am J Clin Dermatol. 2002; 3(6):427–33.
Article10. Slyshenkov VS, Piwocka K, Sikora E, Wojtczak L. Pantothenic acid protects jurkat cells against ultraviolet light-induced apoptosis. Free Radic Biol Med. 2001; Jun. 30(11):1303–10.
Article11. Slyshenkov VS, Rakowska M, Moiseenok AG, Wojtczak L. Pantothenic acid and its derivatives protect Ehrlich ascites tumor cells against lipid peroxidation. Free Radic Biol Med. 1995; Dec. 19(6):767–72.
Article12. Ermis H, Parlakpinar H, Gulbas G, Vardi N, Polat A, Cetin A, et al. Protective effect of dexpanthenol on bleomycin-induced pulmonary fibrosis in rats. Naunyn Schmiedebergs Arch Pharmacol. 2013; Dec. 386(12):1103–10.
Article13. Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978; May. 86(1):271–8.14. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; Mar. 34(3):497–500.
Article15. Aebi H. Catalase. In : Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press;1974. p. 673–7.16. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; Jul. 70(1):158–69.17. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; May. 82(1):70–7.
Article18. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004; Apr. 37(4):277–85.
Article19. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005; Dec. 38(12):1103–11.
Article20. Aycicek A, Erel O, Kocyigit A. Decreased total antioxidant capacity and increased oxidative stress in passive smoker infants and their mothers. Pediatr Int. 2005; Dec. 47(6):635–9.
Article21. Sagit M, Korkmaz F, Akcadag A, Somdas MA. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013; Aug. 270(8):2231–7.
Article22. Kelly TC, Whitworth CA, Husain K, Rybak LP. Aminoguanidine reduces cisplatin ototoxicity. Hear Res. 2003; Dec. 186(1-2):10–6.
Article23. Huang T, Cheng AG, Stupak H, Liu W, Kim A, Staecker H, et al. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci. 2000; Apr-Jun. 18(2-3):259–70.
Article24. Yumusakhuylu AC, Yazici M, Sari M, Binnetoglu A, Kosemihal E, Akdas F, et al. Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol. 2012; Mar. 76(3):404–8.
Article25. Ozturan O, Jerger J, Lew H, Lynch GR, et al. Monitoring of cisplatin ototoxicity by distortion-product otoacoustic emissions. Auris Nasus Larynx. 1996; 23:147–51.
Article26. Ichikawa I, Kiyama S, Yoshioka T. Renal antioxidant enzymes: their regulation and function. Kidney Int. 1994; Jan. 45(1):1–9.
Article27. Kizilay A, Kalcioglu MT, Ozerol E, Iraz M, Gulec M, Akyol O, et al. Caffeic acid phenethyl ester ameliorated ototoxicity induced by cisplatin in rats. J Chemother. 2004; Aug. 16(4):381–7.
Article28. Iraz M, Kalcioglu MT, Kizilay A, Karatas E. Aminoguanidine prevents ototoxicity induced by cisplatin in rats. Ann Clin Lab Sci. 2005; Summer. 35(3):329–35.29. Wojtczak L, Slyshenkov VS. Protection by pantothenic acid against apoptosis and cell damage by oxygen free radicals: the role of glutathione. Biofactors. 2003; 17(1-4):61–73.30. Slyshenkov VS, Dymkowska D, Wojtczak L. Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics. FEBS Lett. 2004; Jul. 569(1-3):169–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphologic Study of Effect of Fosfomycin and Pentoxifylline on Cisplatin Induced Ototoxicity in Guinea Pig
- Preventive Effect of Fosfomycin on Cisplatin Induced Ototoxicity in Cochlea of Guinea Pig
- Protective Effect of Procaine Hydrochloride on Cisplatin Induced Ototoxicity in Guinea Pig
- Cisplatin-Induced Ototoxicity: Updates on Potential Molecular Mechanism
- Nurses’ and Medical Doctors’ Perception and Management Status on Ototoxicity Related to Chemotherapy